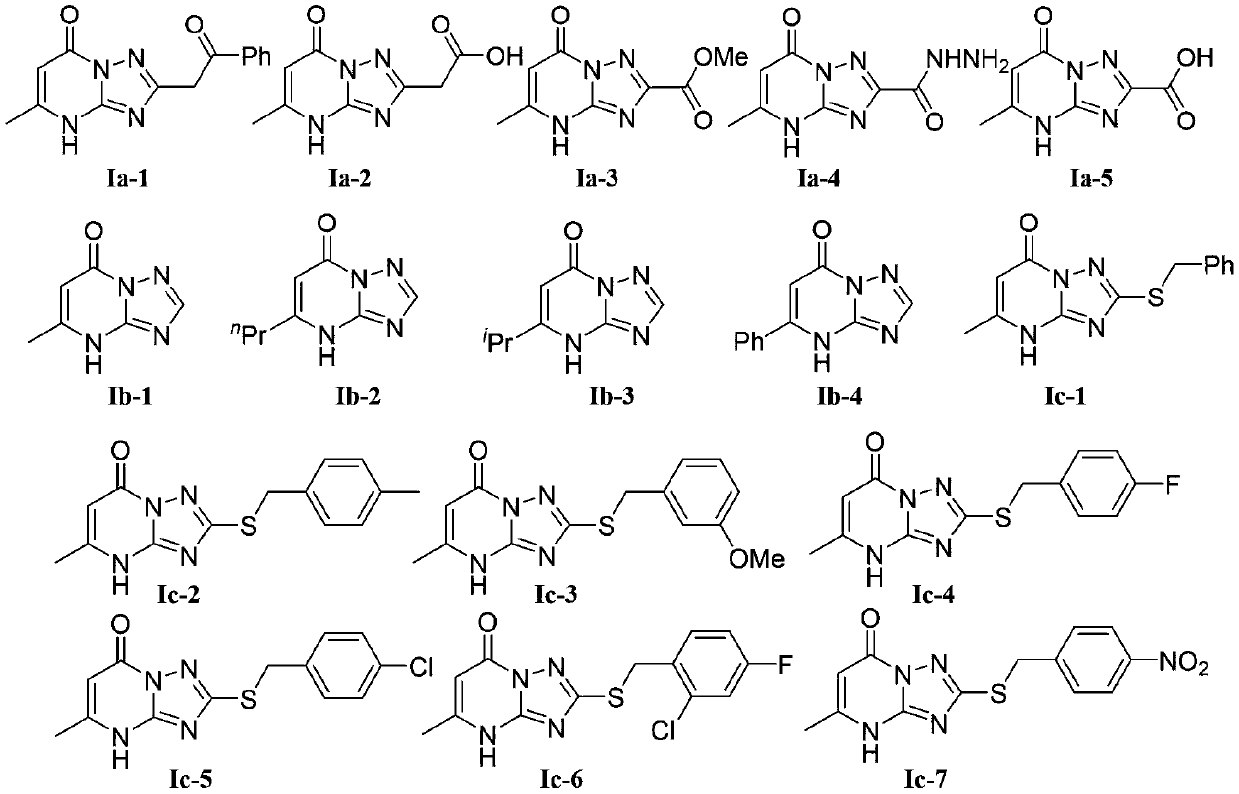
Applications of alkaloid essramycin and derivative thereof in resisting of plant viruses
A technology of anti-plant virus and anti-plant virus agent, applied in the direction of chemicals, applications, and plant growth regulators for biological control, which can solve the problems of low synthesis yield, poor stability and solubility, and achieve expanded application Range, good effect on anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
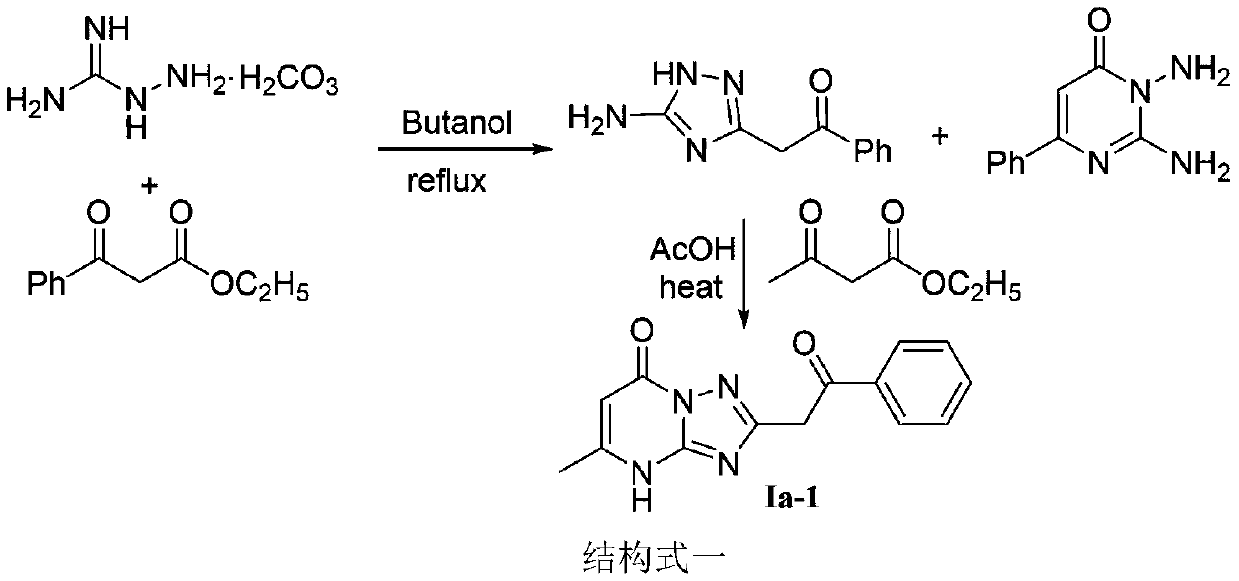
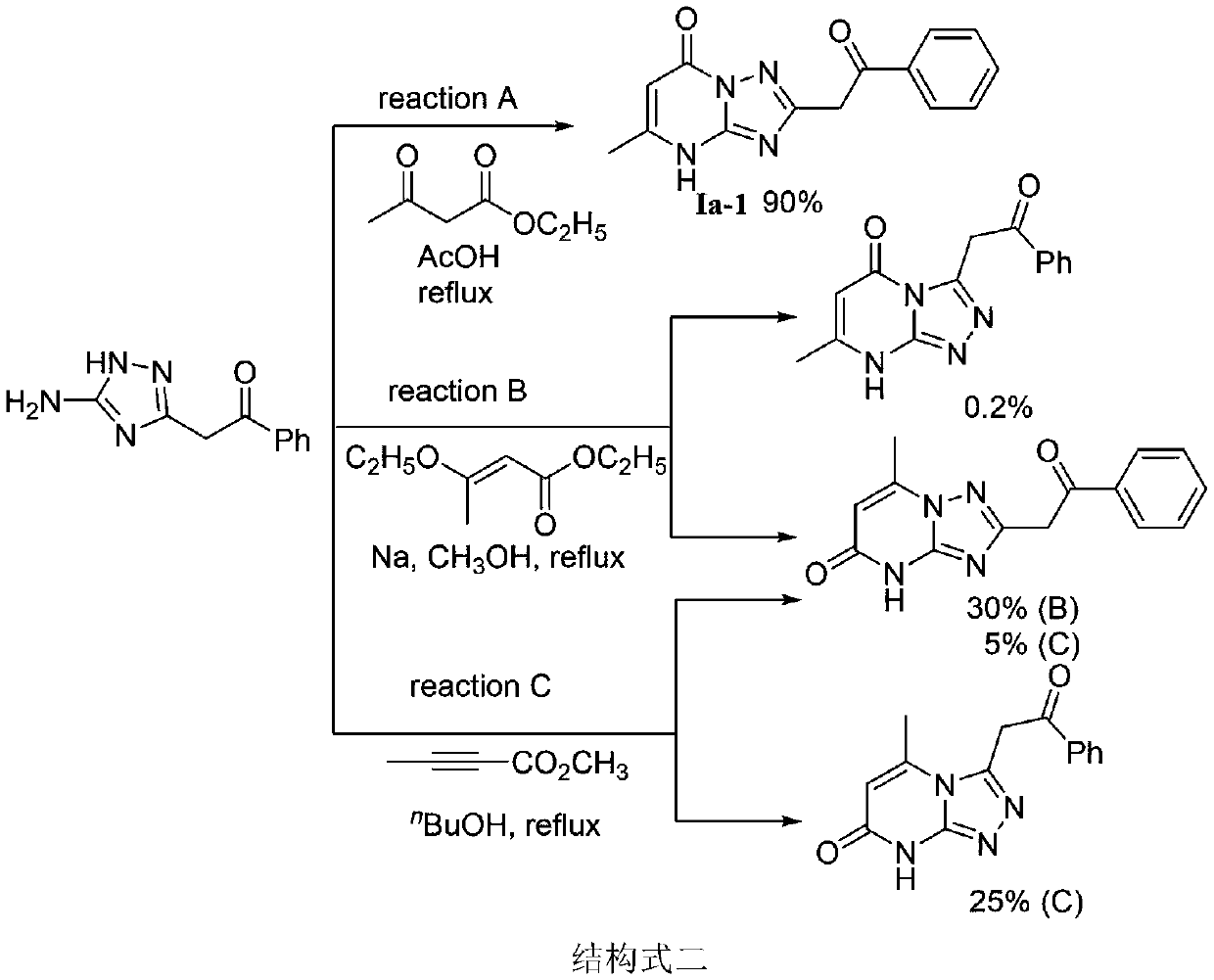
[0022] The preparation method of essramycin shown in chemical structural formula Ia-1 is as follows:
[0023] The chemical structural formula Ia-1 of Essramycin is
[0024]
[0025] The concrete steps of its preparation method are as follows:
[0026] The first step, in the there-necked flask of 250mL, add aminoguanidine bicarbonate (9.64g, 0.07mol), ethyl benzoyl acetate (13.44g, 0.07mol) and n-butanol (40mL) and carry out heating and reflux reaction temperature control at Reflux at 130°C for 3 hours. After the reaction, cool the system to room temperature to precipitate a large amount of light brown solid. After suction filtration and drying, add 50 mL of DMF to the obtained solid product and stir. Stir for more than 30 minutes, and then carry out suction filtration. After washing with DMF and washing with water, the compound 2-(5-amino-1H-1,2,4-triazol-3-yl)-1-phenylethan-1-one was obtained as a white solid, yield 6%, melting point 198°C - 202°C; 1 HNMR (DMSO-d 6 ,40...
Embodiment 2
[0029] 2-(5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazol[1,5-a]pyrimidin-2-yl)acetic acid shown in chemical structure Ia-2 The preparation method is as follows:
[0030] The chemical formula Ia-2 of 2-(5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazol[1,5-a]pyrimidin-2-yl)acetic acid is
[0031]
[0032] The concrete steps of its preparation method are as follows:
[0033] Except replacing 2-(5-amino-1H-1,2,4-triazol-3-yl)-1 with 2-(5-amino-1H-1,2,4-triazol-3-yl)acetic acid -Except for phenylethan-1-one, the others are the same as the second step of Example 1, a white solid with a yield of 97% and a melting point of 257-259°C; 1 H NMR (DMSO-d 6 ,400MHz):12.90(br s,2H,NH),5.82(s,1H,C=CH),3.73(s,2H,CH 2 ),2.32(s,3H,CH 3 ); 13 C NMR (DMSO-d 6 ,100MHz):170.7,159.2,156.0,151.8,151.3,98.7,35.4,19.1; HR-MS(ESI):Calcd for C 8 h 9 N 4 o 3 [M+H] + 209.0669, found (ESI + ) 209.0672, the product was determined to be 2-(5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazol[1,5-a]pyrimidin-2-yl)acet...
Embodiment 3
[0035] The preparation method of 5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazol[1,5-a]pyrimidine-2-carboxylic acid methyl ester shown in chemical structural formula Ia-3 as follows:
[0036] The chemical structure Ia-3 of methyl 5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazol[1,5-a]pyrimidine-2-carboxylate is
[0037]
[0038] The concrete steps of its preparation method are as follows:
[0039] Except replacing 2-(5-amino-1H-1,2,4-triazol-3-yl)-1-phenyl with methyl 5-amino-1H-1,2,4-triazole-3-carboxylate Except for ethyl-1-one, the others are the same as the second step of Example 1, white solid with a yield of 98% and a melting point of 232-234°C; 1 HNMR (DMSO-d 6 ,400MHz):13.39(br s,1H,NH),5.94(s,1H,C=CH),3.91(s,3H,OCH 3 ),2.35(s,3H,CCH 3 ); 13 C NMR (DMSO-d 6 ,100MHz):160.0,155.6,153.0,152.7,151.1,98.7,52.6,18.8; HR-MS(ESI):Calcd for C 8 h 9 N 4 o 3 [M+H] + 209.0669, found (ESI + ) 209.0668, the product was determined to be methyl 5-methyl-7-oxo-4,7-dihydro-[1,2,4]tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com