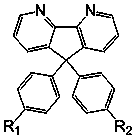
Bipyridine fluorene derivatives, preparation method and application thereof, and electroluminescent device
An electroluminescent device and a technology of dipyridine fluorenes are applied in the field of preparation of dipyridine fluorene derivatives, electroluminescent devices, and can solve the problem of low triplet energy level, weak electron transport ability, and inability to satisfy blue light phosphorescence. devices, etc., to achieve the effect of increasing the glass transition temperature, enhancing the triplet energy level, and being conducive to the development of high-efficiency full-color displays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of 9-phenyl-9-(4,4-dimethyltriphenylamino)-4,5-dipyridinefluorene (MTAPF):
[0058] 9-Phenyl-9-hydroxy-4,5-dipyridinefluorene (1.0 mmol), 4,4′-dimethyltriphenylamine (1.2 mmol) were dissolved in dry dichloromethane (20 ml), while Add trifluoromethane acid (1.0 mmol) under nitrogen atmosphere, heat to 110°C and reflux for 24 hours in the dark. Then cool to room temperature, add water to quench the reaction, extract with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, remove the organic solvent, and recrystallize with dichloromethane and methanol to obtain white solid powder 9-phenyl-9- (4,4-Dimethyltriphenylamino)-4,5-dipyridinefluorene (MTAPF). Yield: 59%. Mass Spectrum (Mass Spectrum) MS (APCI): calcd for C37H29N3: 515.2, found, 516.4 (M+1)+.
Embodiment 2
[0060] Preparation of 4-methyl-4′,4″-bis(9-phenyl-4,5-diaza-9-fluorenyl)triphenylamine (TADPF):
[0061] Using a method similar to the compound MTAPF, the difference is that 4-methyltriphenylamine is used instead of 4,4'-dimethyltriphenylamine as the starting material, and the ratio of raw materials is changed, 9-phenyl-9-hydroxyl- The molar mass ratio of 4,5-dipyridine fluorene to 4-methyltriphenylamine and trifluoromethane acid is 2.5: 1: 2.5. 4-Methyl-4′,4″-bis(9-phenyl-4,5-diaza-9-fluorenyl)triphenylamine (TADPF) white solid powder can be obtained, yield: 64%. Mass spectrum MS (APCI): calcd for C53H37N5: 743.3, found, 744.4 (M+1)+.
Embodiment 3
[0063] Preparation of 9,9-bis(4,4′-dimethyltriphenylamino)dipyridylfluorene (DTAPF):
[0064] Using a method similar to the compound MTAPF, the difference is that 9-phenyl-9-hydroxyl-4,5-dipyridine fluorene is used as the starting material instead of 9-phenyl-9-hydroxy-4,5-dipyridine fluorene, and 4,4′-bispyridine fluorenone is combined with 4,4′-dipyridine The molar mass ratio of methyltriphenylamine and trifluoromethane is: 1: 2.5: 2. 9,9-bis(4,4′-dimethyltriphenylamino)dipyridylfluorene (DTAPF) white solid powder can be obtained, yield: 90%. Mass MS (APCI): calcd for C51H42N4: 710.3, found, 711.3 (M+1)+.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com