Monoclonal antibodies against claudin-18 for treatment of cancer
A technology of antibodies and cancer cells, applied in the direction of anti-animal/human immunoglobulins, antibodies, immunoglobulins, etc., can solve problems such as inaccessibility of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0501] 1. Generation of mouse antibodies against CLD18
[0502] a. Immunity:
[0503] Balb / c or C57 / BL6 mice were immunized with eukaryotic expression vectors encoding human CLD18 fragments (SEQ ID NO: 15, 16, 17, 18). In the presence of an adjuvant (such as CpG), 50 μg or 25 μg of plasmid DNA was injected into the quadriceps muscle (intramuscular, i.m.) on days 1 and 10 for the production of group 1 monoclonal antibodies, or on day 1 Day 1 and day 9, day 1 and day 11 or day 1, day 16 and day 36 for generation of a second set of antibodies (see Table 1b for details). CpG was injected intramuscularly along with cells transfected with CLD18A2 (SEQ ID NO: 1 ) alone or co-transfected with CLD18A2 and mouse soluble CD40L encoding RNA, PEI-Man was injected intramuscularly or intraperitoneally. Depending on the specific immunization protocol used, mouse sera were monitored for the presence of antibodies against human CLD18 by immunofluorescence microscopy between days 16 and 43. I...
example
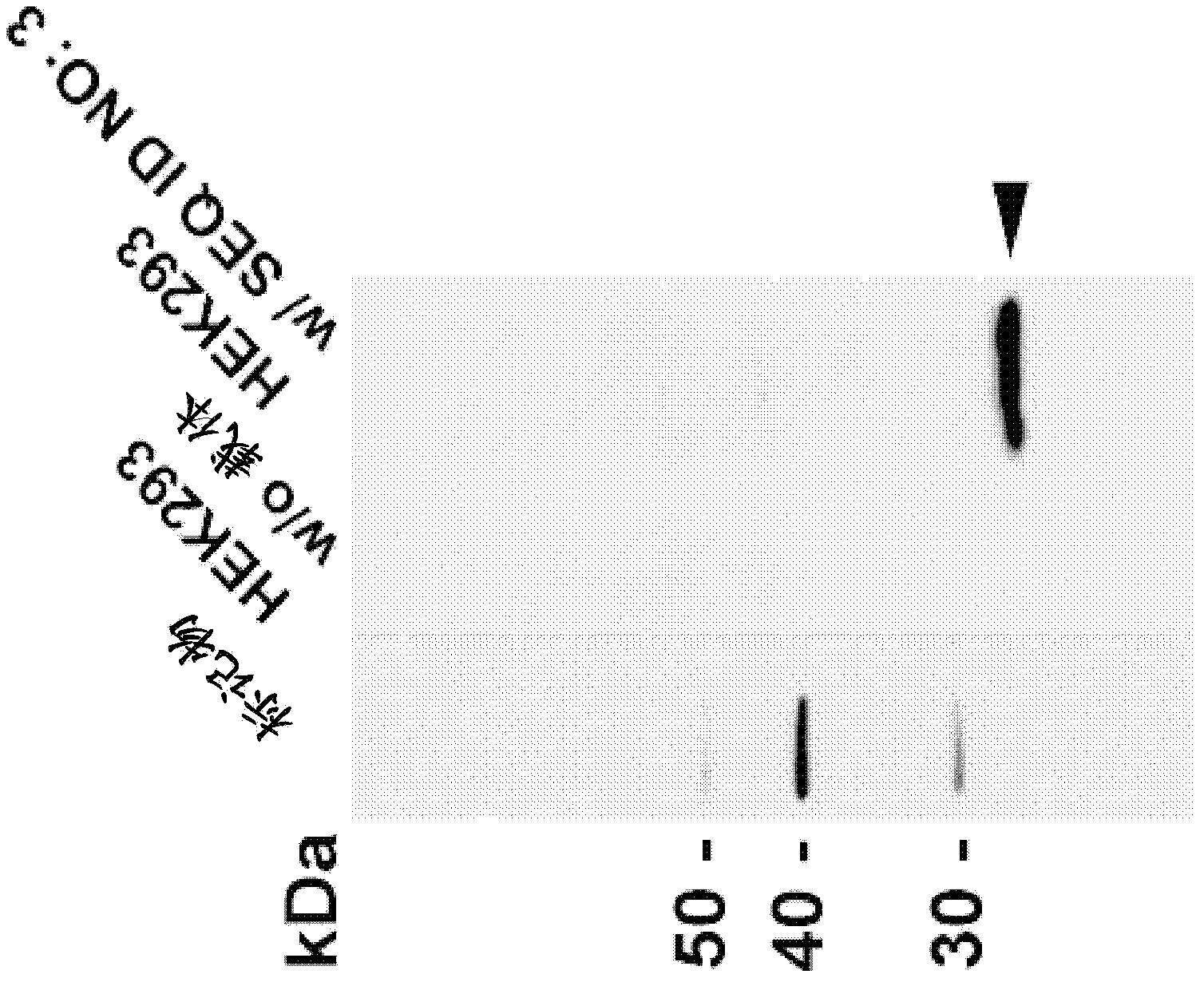
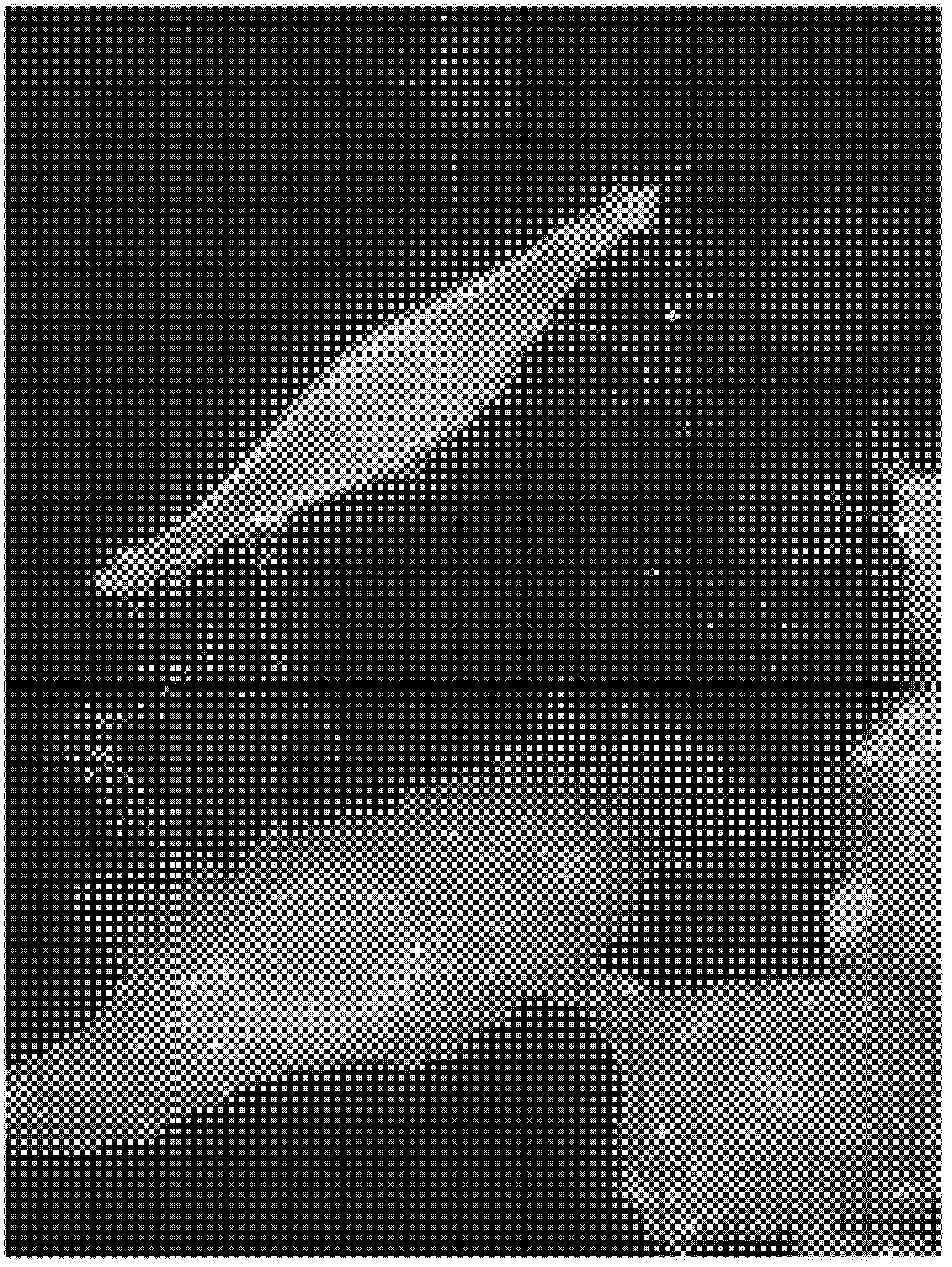
[0551] The binding specificity of the identified mAbs to the CLD18A2 isoform was analyzed by flow cytometry. HEK293 cells stably expressing human CLD18A2 (HEK293-CLD18A2) and HEK293 cells stably expressing human CLD18A1 (SEQ ID NO: 7, 8) (HEK293-CLD18A1) were incubated with hybridoma supernatants containing monoclonal antibodies at 4°C After 30 minutes incubation with Alexa647-conjugated anti-mouse IgG secondary antibody and cells fixed or left unfixed and counterstained with PI. Binding was assessed by flow cytometry using a BD FACSArray. Figure 6 shows the Examples of two groups of mAbs identified in 175D10: (i) mAbs 43A11, 45C1 and 163E12 specifically bind human CLD18A2, but not human CLD18A1 ( Figure 6A , B), and (ii) mAb 37H8 binds to both human isoforms ( Figure 6A ).
[0552] e. Comparison of antibody binding to human CLD18A1 and CLD18A2 transfectants by immunofluorescence microscopy:
[0553]HEK293 cells were transiently transfected with an expression vector enc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com