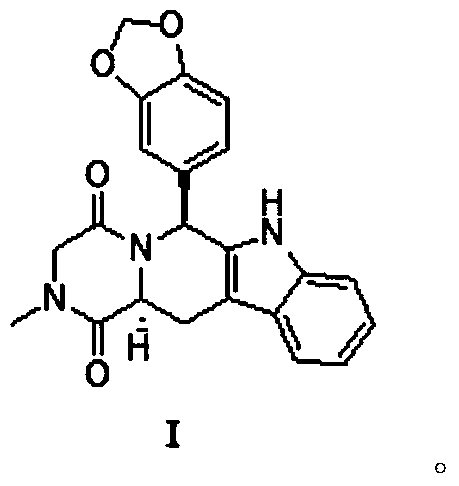
Preparation method of tadalafil
A compound and solvent technology, applied in the field of medicine, can solve problems to be improved, and achieve the effects of short production cycle, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
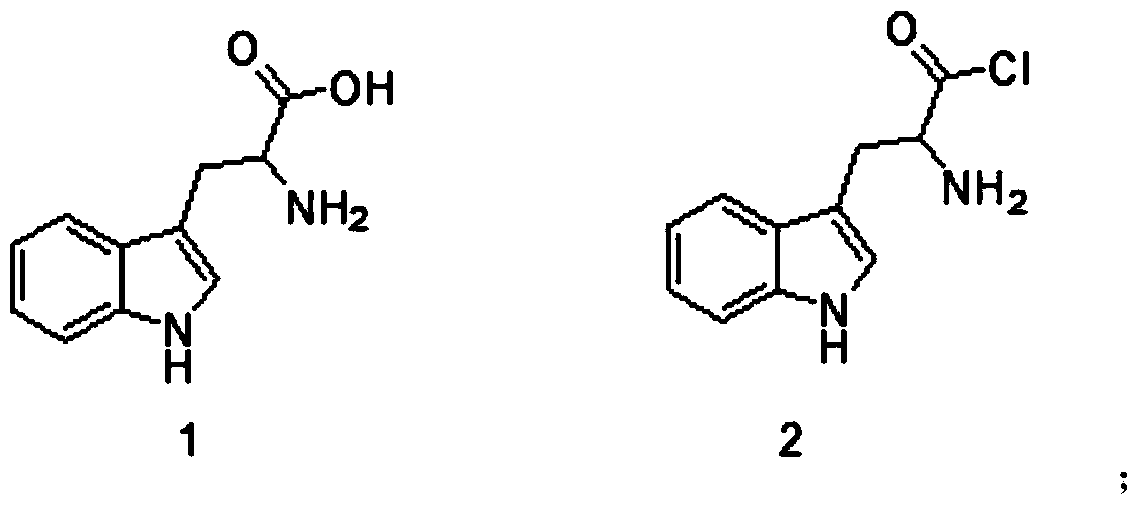
[0042] Weigh 100g compound 1 into a 2L three-necked flask, stir, add 1000mL dichloromethane and 117g SOCl 2 , 5mL DMF is heated to 40 degrees Celsius. After reacting for 1 hour, it was cooled and spin-dried to obtain 128 g of compound 3. The crude yield was> 99%. Melting point: 230 to 232 degrees Celsius (decomposition).
Embodiment 2
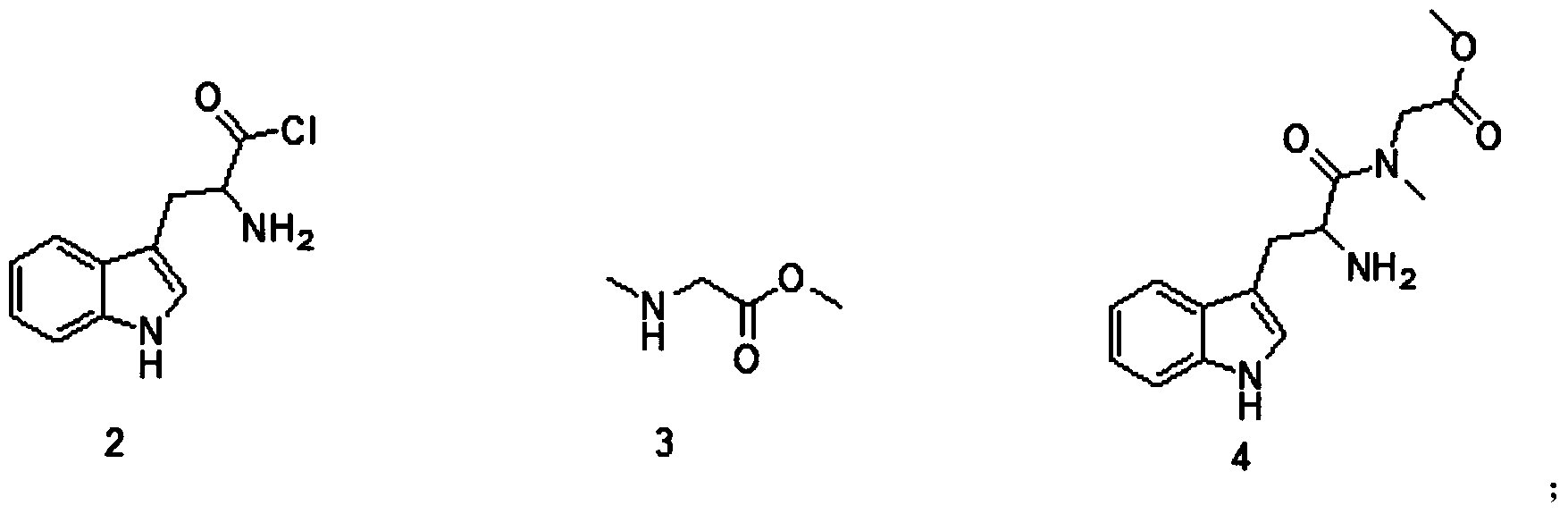
[0044] Place 100g of compound 3 in a 2L three-necked flask, add 1000mL of dichloromethane, stir, and cool to 0 degrees Celsius. After compound 3 is dissolved, add 146g of diisopropylethylamine, slowly add 222g of compound 2, and keep it after adding. React at 0 degrees Celsius for 10 minutes, raise the temperature to 25 degrees Celsius, stir and react for 4 hours, stop the reaction, add dichloromethane and water, separate layers, and extract. The organic phase was washed three times with water, three times with saturated brine, dried, concentrated and added with 12N concentrated hydrochloric acid to make the product salted out, and filtered to obtain 220.2 g with a yield of 94%. 1 H NMR(CDCl 3 ): δ8.25(s,1H), 7.47(d,J=7.6Hz,1H), 7.29(m,2H), 7.00-7.19(m,3H), 5.65(d,J=8.3Hz,1H) ,4.95-5.07(m,3H),4.05(d,J=17.3Hz,1H), 3.88(d,J=17.2Hz,1H), 3.69(s,3H), 3.09-3.16(m,2H), 2.86(s,1H); MS(m / z): 326.1[M+H] + .
Embodiment 3
[0046] Put 100g of compound 3 in a 2L three-necked flask, add 1000mL of dichloromethane, stir, and cool to 0 degrees Celsius. After compound 3 is dissolved, add 108g of triethylamine, slowly add 222g of compound 2, and keep at 0 degrees Celsius after the addition. Reaction 10 Minutes, the temperature was raised to 25 degrees Celsius, the starting material was observed to disappear, the reaction was stopped, EA and water were added, layered, and extracted. The organic phase was washed three times with water, three times with saturated brine, dried, concentrated and added with 12N concentrated hydrochloric acid to make the product salted out, filtered to obtain compound 4 with a mass of 164 g and a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com