Method for catalyzed synthesis of N-(phosphonomethyl) iminodiacetic acid by p-toluenesulfonic acid
A technology of p-toluenesulfonic acid and diglyphosate, which is applied in the chemical industry, can solve the problems of decreased yield of diglyphosate, volatile loss, poor operating environment, etc., and achieve the effects of reducing production costs, high solubility, and reducing material loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
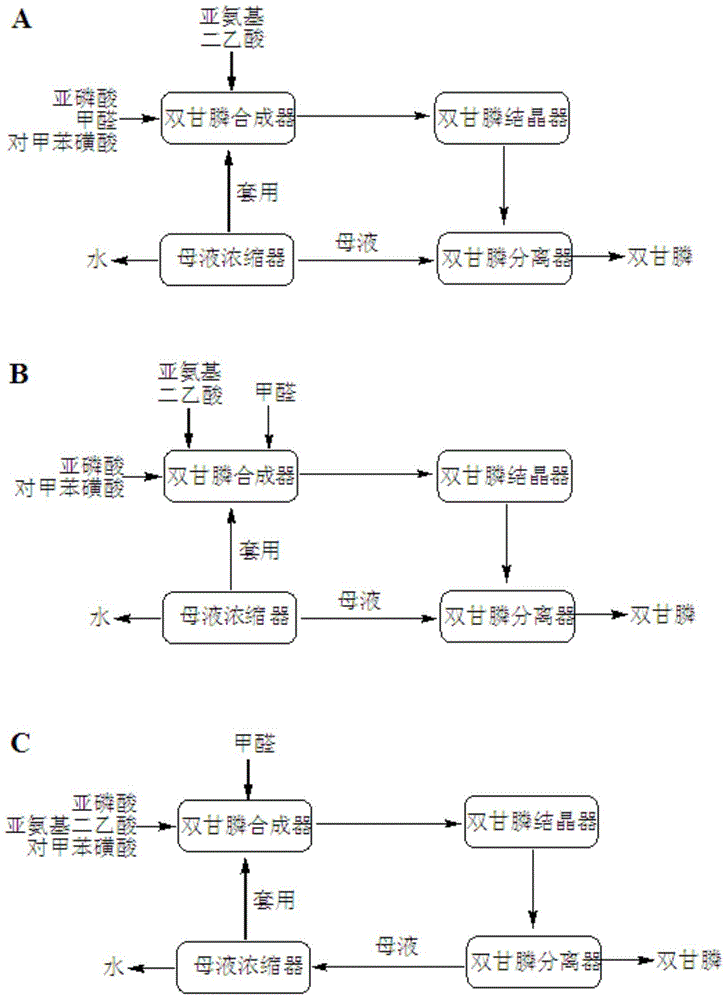
[0026] Add phosphorous acid 91.6g (content 98.5%, 1.1mol), formaldehyde 97.3g (content 37%, 1.2mol), p-toluenesulfonic acid monohydrate 9.5g (content 99%, 0.05mol) and water 200mL into the reactor, Heat to 115-120°C, add a suspension consisting of 135.7g of iminodiacetic acid (content 98%, 1mol) and water 300mL dropwise at a constant speed within 3 hours, and keep it warm for 2 hours after adding; transfer the reaction solution to the crystallizer In the medium, lower the temperature to 10-15°C, heat and crystallize for 2 hours; then transfer the solid-containing liquid to a separator, separate the liquid from the solid, wash the solid with 20 mL of water, and dry to obtain 210.0 g of bisglyphosate (white solid), with a content of 98.7% , yield 91.3%; mother liquor weighs 603g, which contains 8.9g p-toluenesulfonic acid.
[0027] Transfer the mother liquor to the concentrator, concentrate to remove 140g of water, and then send it back to the reactor, add 0.6g of p-toluenesulfo...
Embodiment 2
[0030] Add 150.4g of phosphorous acid (content 60%, 1.1mol), 19.2g of p-toluenesulfonic acid monohydrate (content 99%, 0.1mol) and 300mL of water into the reactor, heat to 115-120°C, within 3 hours at the same time Add formaldehyde 97.3g (content 37%, 1.2mol) and a suspension composed of iminodiacetic acid 135.7g (content 98%, 1mol) and water 300mL dropwise at a uniform speed, and keep warm for 2 hours after adding; transfer the reaction solution to the crystallization In the container, cool down to 10-15°C, heat and crystallize for 2 hours; then transfer the solid-containing liquid to the separator, separate the liquid from the solid, wash the solid with 20 mL of water, and dry to obtain 215.7 g of bisglyphosate (white solid), with a content of 98.3 %, the yield is 93.4%; the mother liquor weighs 607g, which contains 9.3g of p-toluenesulfonic acid.
[0031] Transfer the mother liquor to the concentrator, concentrate to remove 250g of water, then return to the reactor, add 10....
Embodiment 3
[0034] Add 135.7g of iminodiacetic acid (content 98%, 1mol), phosphorous acid 91.6g (content 98.5%, 1.1mol), 201.5g of p-toluenesulfonic acid monohydrate (content 99%, 1.05mol) and water into the reactor 500mL, heat to 115-120°C, add formaldehyde 97.3g (content 37%, 1.2mol) dropwise at a uniform speed, and keep warm for 2 hours after adding; hour; then transfer the solid-containing liquid to the separator, separate the liquid from the solid, wash the solid with 20mL of water, and dry to obtain 212.0g of bisglyphosate (white solid), with a content of 98.5% and a yield of 92.0%; the weight of the mother liquor is 792.0g, of which Contains 199.6g of p-toluenesulfonic acid.
[0035] Transfer the mother liquor to the concentrator, concentrate to remove 42g of water, and then send it back to the reactor, add 2.0g of p-toluenesulfonic acid monohydrate, and add 135.7g of iminodiacetic acid (content 98%, 1mol) and phosphorous acid 87.4g (content 98.5%, 1.05mol), heated to 115-120°C, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com