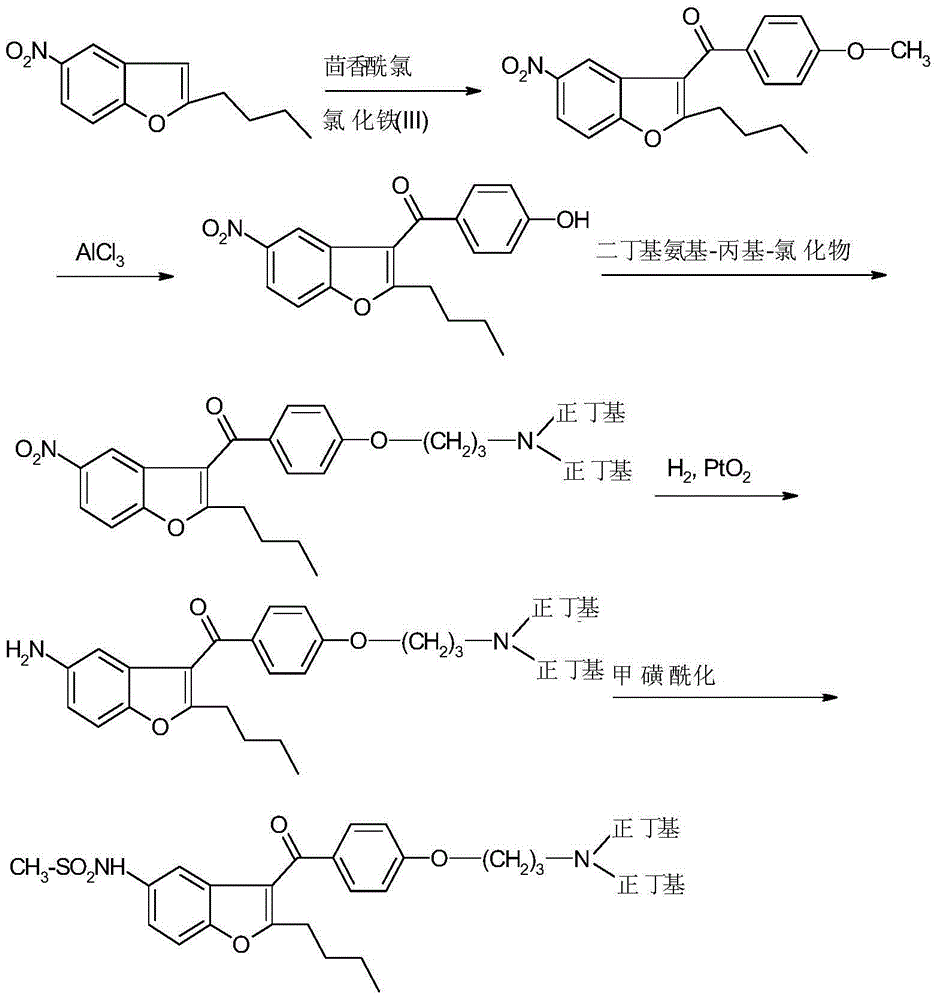
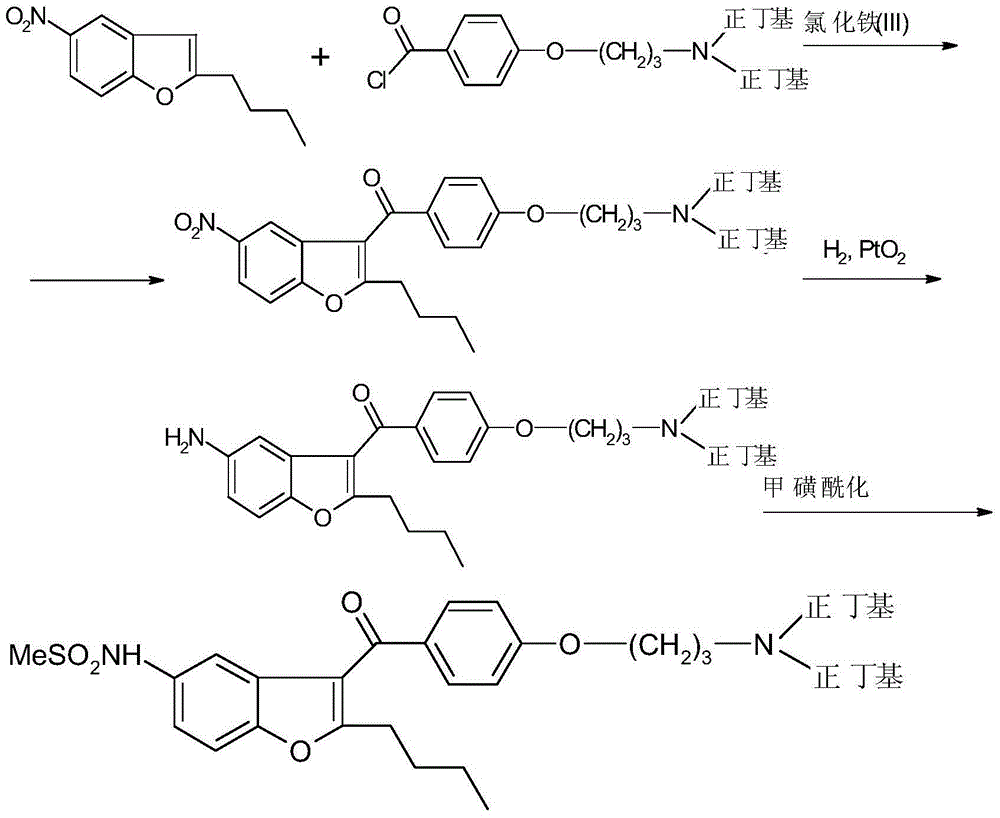
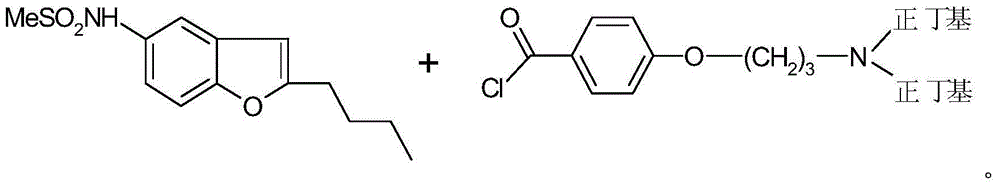
Reductive amination process for preparation of dronedarone using amine intermediary compound
A technology of compounds and reducing agents, applied in the fields of organic chemistry, drug combination, cardiovascular system diseases, etc., can solve the problem that the steps cannot be purified.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] N-[2-Butyl-3-{4-[(3-dibutylamino)propoxy]benzoyl}-1-benzofuran-5-yl]methanesulfonamide (I)
[0169] Dissolve 1 g of N-[2-butyl-3-{4-[(3-amino)propoxy]benzoyl}-1-benzofuran-5-yl]-methanesulfonamide in 30 ml of dichloromethane middle. 0.5 g of butyraldehyde and 1.8 g of triacetoxyborohydride were added, and the reaction mixture was stirred at 20° C. for 12 hours. The reaction mixture was evaporated and the residue was dissolved in isopropyl acetate. Dilute the solution with 20ml water, 10ml5%NaHCO 3 solution and washed with 10ml of water. The solvent was evaporated.
[0170] Yield: 1.21g (94.5%).
[0171] The product was purified by forming its oxalate salt as follows: 6 ml methyl ethyl ketone was added to the residue and the mixture was heated to 70°C. To this solution was added 0.26 g of oxalic acid dissolved in 2.5 ml of methyl ethyl ketone at 70°C. Cool to 20°C over 6 hours, then stir the mixture at 10°C for 1 hour and filter. To the resulting oxalate was adde...
Embodiment 2
[0176] N-[2-Butyl-3-{4-[(3-dibutylamino)propoxy]benzoyl}-1-benzofuran-5-yl]methanesulfonamide (I)
[0177] 1 g of N-[2-butyl-3-{4-[(3-amino)propoxy]benzoyl}-1-benzofuran-5-yl]methanesulfonamide was dissolved in 12 ml of butyric acid. 0.2 g of butyraldehyde and 0.26 g of sodium borohydride were added. The mixture was stirred at 55°C for 8 hours. Cool to 0°C and add 20ml of water. The mixture was made strongly basic with solid potassium hydroxide and extracted with 2x20ml dichloromethane. Dilute the solution with 25ml of water, 15ml of 5%NaHCO 3 Washed with 10ml of water and evaporated.
[0178] Yield: 1.10g (85.9%)
[0179] The product was purified according to Example 1 by its oxalate salt (87%).
[0180] Product purity: 99.6% (HPLC). The product was identical to the compound prepared in Example 1.
Embodiment 3
[0182] N-[2-Butyl-3-{4-[(3-dibutylamino)propoxy]benzoyl}-1-benzofuran-5-yl]methanesulfonamide (I)
[0183] 1 g of N-[2-butyl-3-{4-[(3-amino)propoxy]benzoyl}-1-benzofuran-5-yl]methanesulfonamide was dissolved in 12 ml of butyric acid and 0.39 g sodium borohydride was added. The mixture was stirred at 55°C for 8 hours. Cool to 0°C and add 20ml of water. The mixture was made strongly basic with solid potassium hydroxide and extracted with 2x20ml dichloromethane. Dilute the solution with 25ml of water, 15ml of 5%NaHCO 3 Washed with 10ml of water and evaporated.
[0184] Yield: 0.88g (69%)
[0185] The product was purified according to Example 1 by its oxalate salt (81%).
[0186] Product purity: 99.4% (HPLC). The product was identical to the compound prepared in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com