Application of bismuth phosphate compound serving as catalyst for photochemical water splitting hydrogen production
A technology of photolysis of water to produce hydrogen and compounds, applied in the field of catalysis, can solve the problems of increasing catalyst cost, difficult batch controllable preparation of catalysts, etc., and achieve the effect of excellent hydrogen production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Using bismuth chloride, sodium phosphate, and ethanol as starting materials, weigh 100mmol of bismuth chloride and 100mmol of sodium phosphate, and dissolve the two in 1000mL of ethanol; stir for 12 hours to fully react, and then separate the reacted solution A precipitate was obtained; the obtained precipitate was washed 10 times with deionization, and dried at 60° C. for 4 hours to obtain the final product.
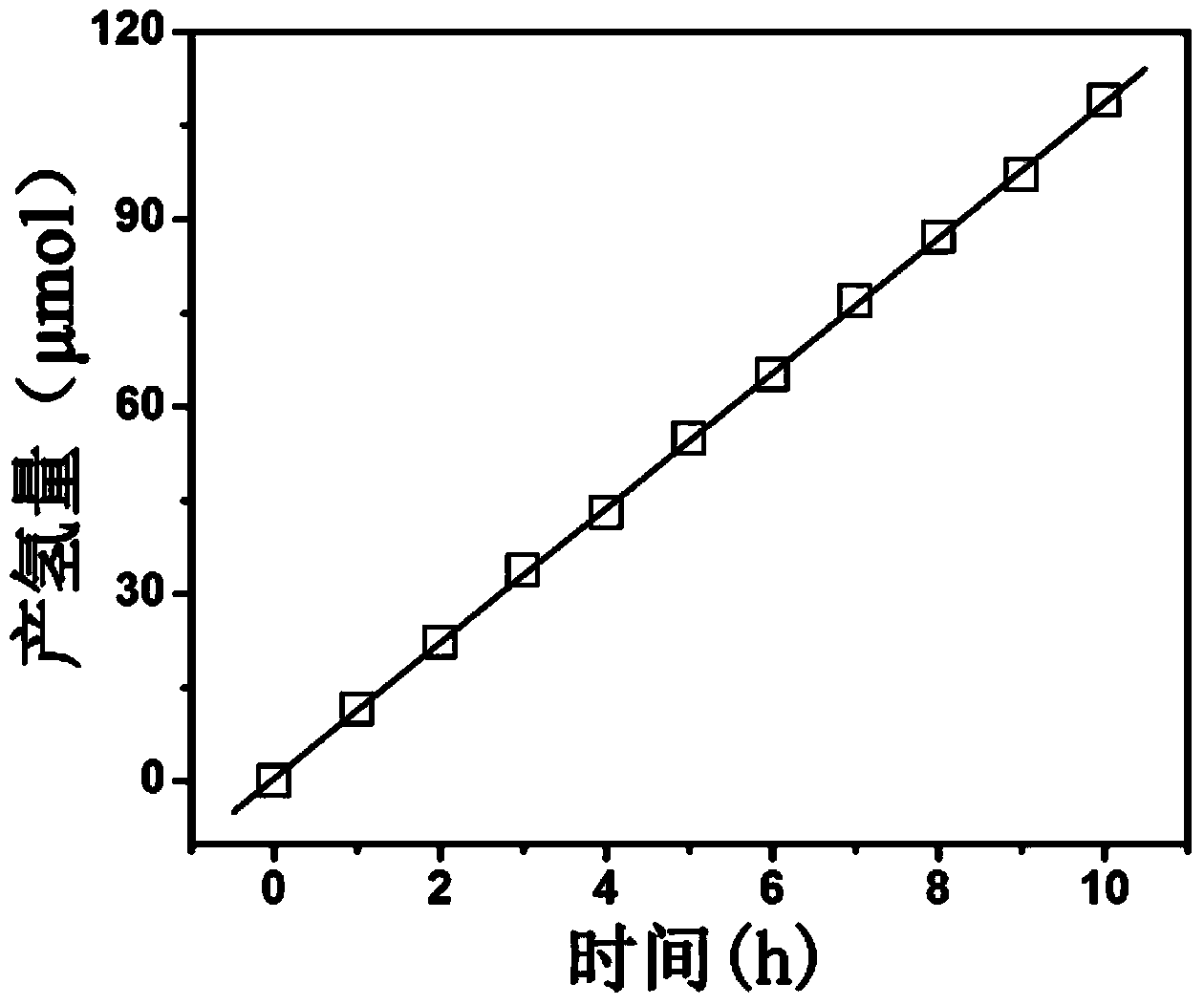
[0014] The bismuth phosphate compound can be used as a catalyst for hydrogen production by photolysis of water. According to the above test conditions for hydrogen production by photolysis of water, the obtained performance curve of hydrogen production by photolysis of water is as follows figure 1 shown. It can be seen from the figure that the hydrogen production rate is 10.8 μmol h -1 .
Embodiment 2
[0016] Using bismuth nitrate, ammonium dihydrogen phosphate, and ethylene glycol as starting materials, weigh 100mmol of bismuth nitrate and 100mmol of ammonium dihydrogen phosphate, and dissolve the two in 100mL of ethylene glycol; stir for 12 hours to fully react, Then the reacted solution was separated to obtain a precipitate; the obtained precipitate was washed 10 times with deionization, and dried at 60° C. for 4 hours to obtain a final product.
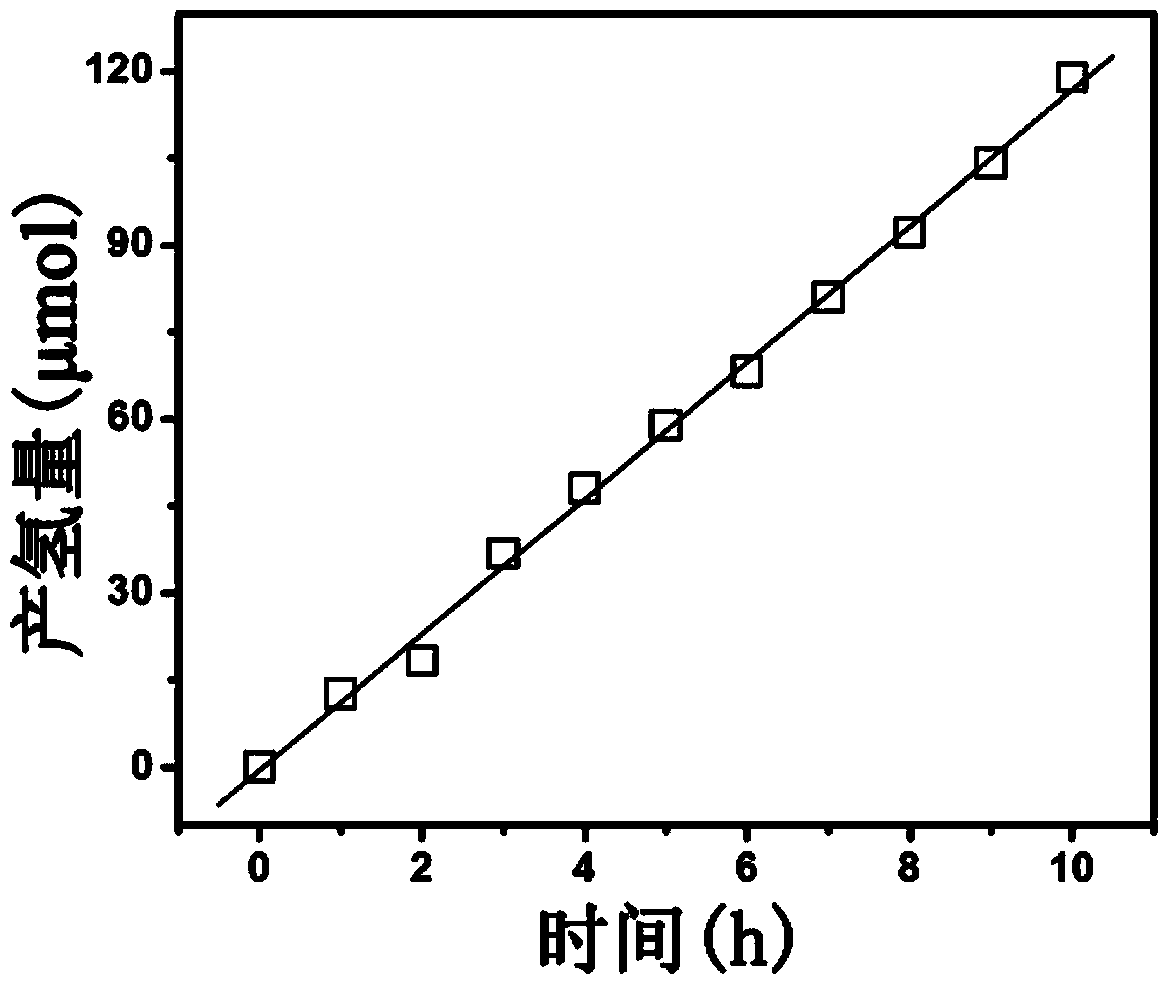
[0017] The bismuth phosphate compound can be used as a catalyst for hydrogen production by photolysis of water. According to the above test conditions for hydrogen production by photolysis of water, the obtained performance curve of hydrogen production by photolysis of water is as follows figure 2 shown. It can be seen from the figure that the hydrogen production rate is 11.3 μmol h -1 .
Embodiment 3
[0019] Taking bismuth nitrate, potassium phosphate, and ethylene glycol methyl ether as starting materials, weigh 100mmol of bismuth nitrate and 100mmol of potassium phosphate, and dissolve the two in 1000mL of ethylene glycol methyl ether; stir for 12 hours to make it fully react, and then The reacted solution was separated to obtain a precipitate; the obtained precipitate was washed 10 times with deionization, and dried at 60° C. for 4 hours to obtain the final product.
[0020] The bismuth phosphate compound can be used as a catalyst for hydrogen production by photolysis of water. According to the above test conditions of hydrogen production by photolysis of water, the performance curve of hydrogen production by photolysis of water was obtained. Analysis showed a hydrogen production rate of 9.6 μmol h -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com