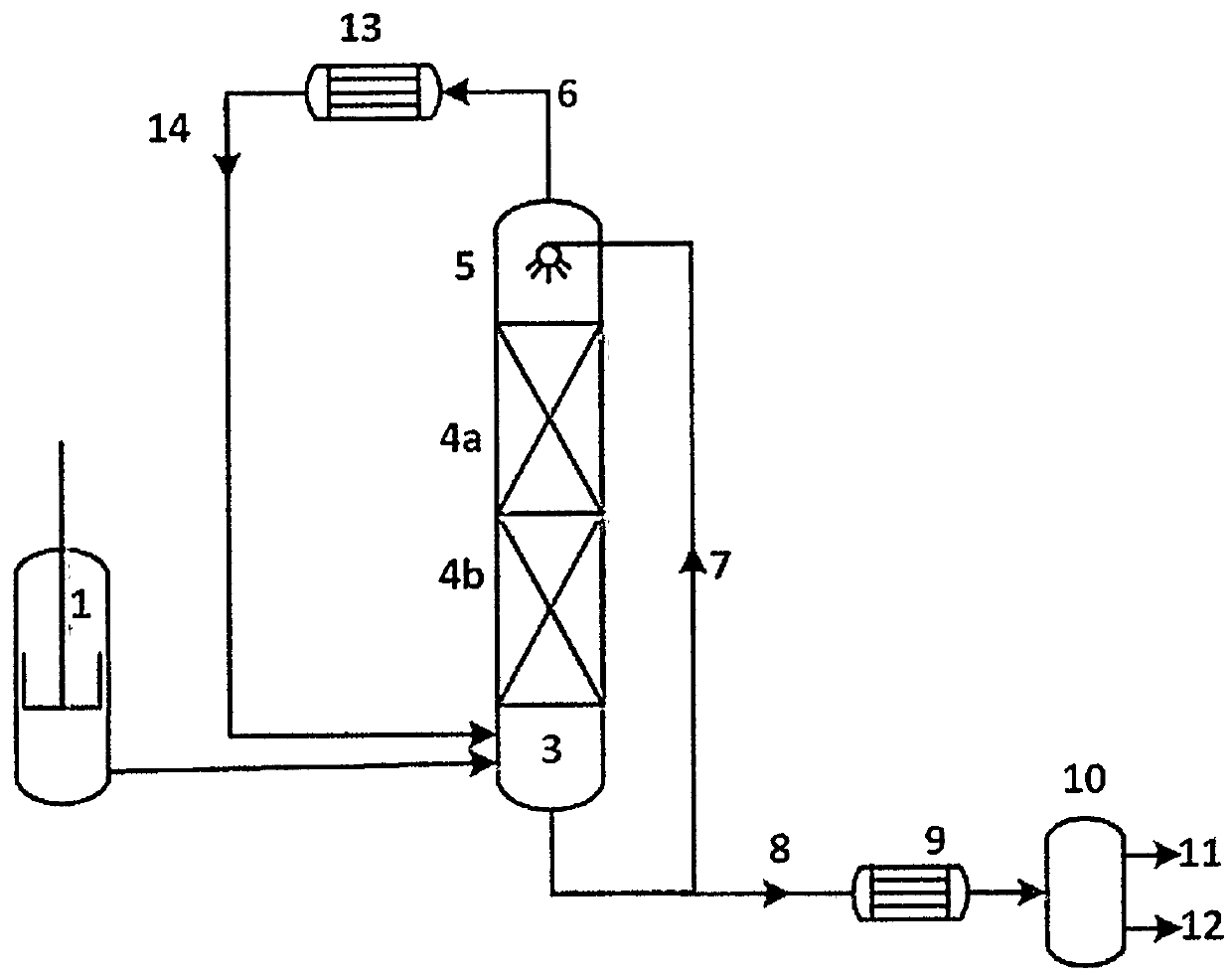
Method for synthesizing 2-methallyl alcohol through continuous hydrolysis reaction
A technology of methallyl alcohol and hydrolysis reaction, which is applied in the fields of hydrolysis preparation, chemical instruments and methods, and preparation of organic compounds, and can solve the problems of low production cost, high selectivity of 2-methallyl alcohol, and 2-methacryl alcohol. Problems such as high conversion rate of allyl chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The reaction conditions are:
[0032] The raw material mixture is composed of 2-methallyl chloride in mass ratio: alkali: water: phase transfer catalyst is 1: 0.4: 3: 0.02; the alkali is sodium hydroxide, and the phase transfer catalyst is tetrabutylammonium bromide; the reaction The pressure of the reactor is 60KPa in terms of gauge pressure; the temperature of the reactor is 95° C.; the residence time in the reactor is 18 hours.
[0033] The result of the reaction is:
[0034] The conversion rate of 2-methallyl chloride is 88.9%;
[0035] The selectivity to 2-methallyl alcohol was 94.2%.
Embodiment 2
[0037] The reaction conditions are:
[0038] The raw material mixture is composed of 2-methallyl chloride in mass ratio: alkali: water: phase transfer catalyst is 1: 0.3: 1: 0.001; the base is sodium hydroxide, and the phase transfer catalyst is tetrabutylammonium bromide; the reaction The pressure of the reactor is 100KPa in terms of gauge pressure; the temperature of the reactor is 100° C.; the residence time in the reactor is 32 hours.
[0039] The result of the reaction is:
[0040] The conversion rate of 2-methallyl chloride is 90.8%;
[0041] The selectivity to 2-methallyl alcohol was 93.2%.
Embodiment 3
[0043] The reaction conditions are:
[0044] The raw material mixture is composed of 2-methallyl chloride in mass ratio: alkali: water: phase transfer catalyst is 1: 0.5: 5: 0.003; the alkali is sodium hydroxide, and the phase transfer catalyst is tetrabutylammonium bromide; the reaction The pressure of the reactor is 10KPa in terms of gauge pressure; the temperature of the reactor is 90° C.; the residence time in the reactor is 4 hours.
[0045] The result of the reaction is:
[0046] The conversion rate of 2-methallyl chloride is 89.3%;
[0047] The selectivity to 2-methallyl alcohol was 94.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com