Novel useful active agent for preventing and/or treating dandruff on the scalp
A dandruff, scalp technology, applied in the field of new beneficial active agents for the prevention and/or treatment of dandruff conditions of the scalp, capable of solving problems such as non-adherence to treatment, slowing down of development, effects of dandruff active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Preparation of the active agent according to the invention
[0169] The complete fermentation medium was prepared by culturing the strain of Vitiligo linearis in its complete medium.
[0170] The initial medium used to obtain the complete fermentation medium had the composition described in Table 1 below.
[0171] Table 1
[0172] Chemical Name
[c]
Autolyzed Extract of Yeast
4g / l
[0173] Soy Papaya Peptone F
3g / l
Dextrose - Dextrose Monohydrate (Roferose)
3g / l
K H 2 PO 4
0.088g / l
CaCl 2
0.050g / l
CuSO 4 ·5H 2 o
60μg / l
MnSO 4 ·1H 2 o
152μg / l
KI
20μg / l
ZnSO 4 ·7H 2 o
200μg / l
AlCl 3 ·6H 2 o
100μg / l
seepage water
Surplus 1l
[0174] In order to obtain the cosmetic active agent according to the invention, ie in this example the lysate of Vitiligo vitratus in complete fermentation medium, the method ...
Embodiment 2
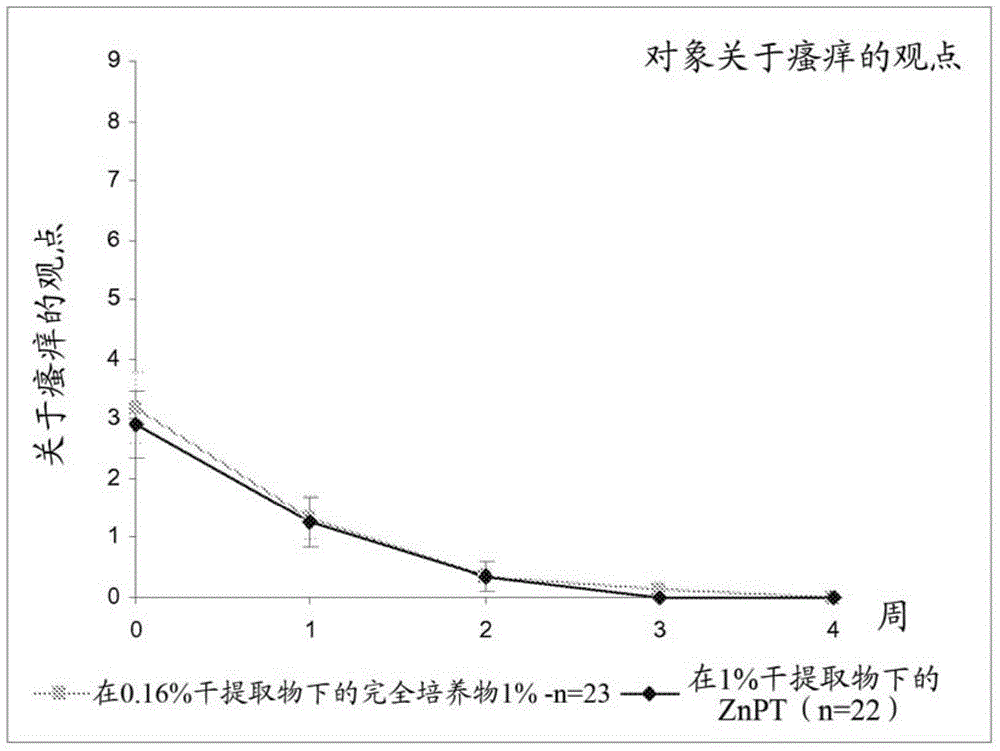
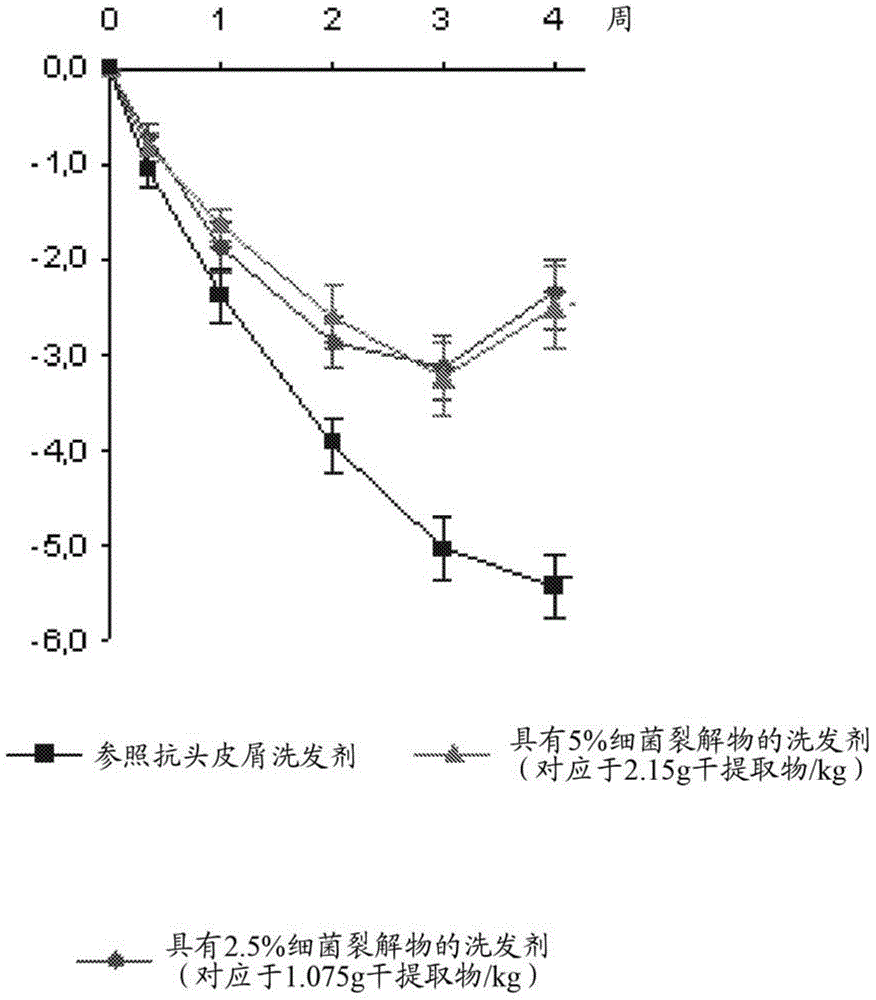
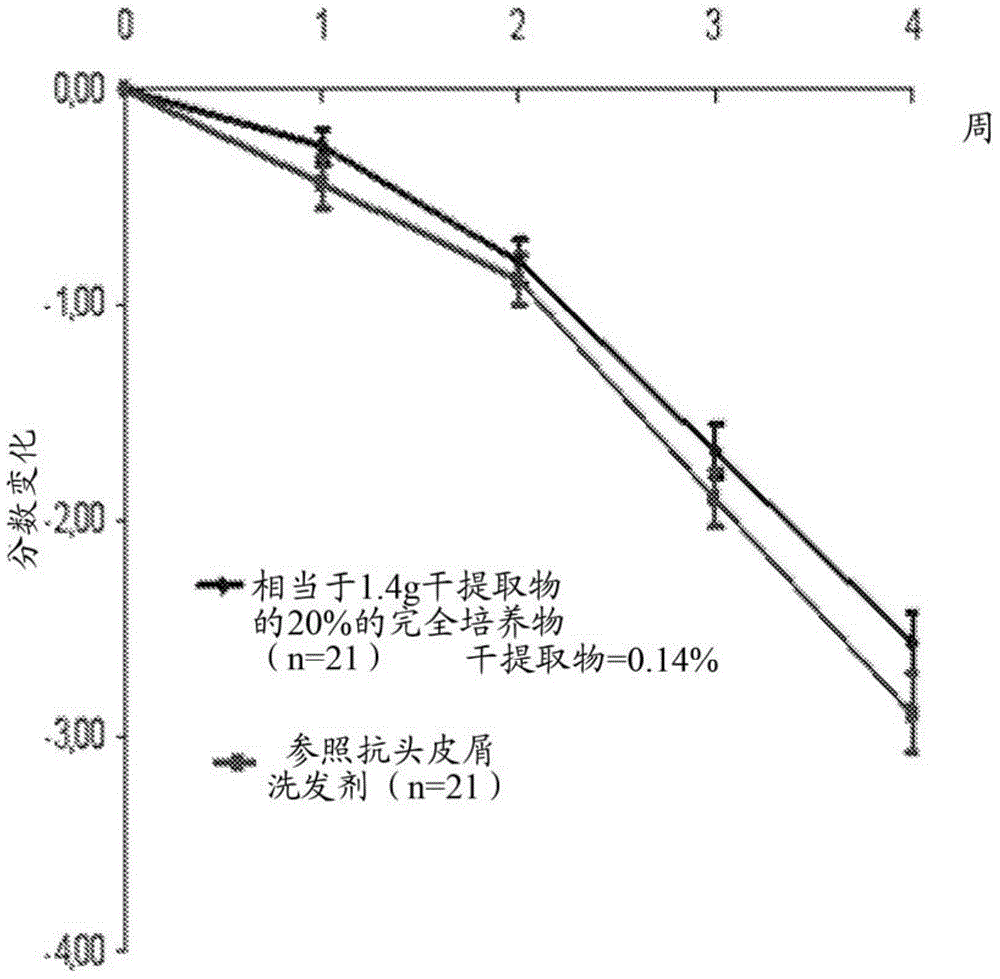
[0187] In Example 2, (i) shampoos comprising an anti-dandruff active agent according to the invention (a lysate of Vitiligo vibriella bacteria in a complete fermentation medium prepared according to Example 1) were compared Effect of formulations and (ii) reference anti-dandruff shampoo formulations without anti-dandruff active agents according to the invention and comprising conventional anti-dandruff active agents (ZnPT) on dandruff conditions of the scalp.
[0188] Therefore, the lysate of the bacteria in the complete fermentation medium (anti-dandruff active agent according to the invention) as obtained in Example 1 was used as anti-dandruff active agent in a shampoo formulation, which The basic composition of the dosage formulation is described in Table 2 below.
[0189] Several clinical studies have been conducted,
[0190] Clinical Study 1, corresponding to figure 1 ,
[0191] Clinical Study 2, corresponding to figure 2 ,
[0192] Clinical Study 3, corresponding ...
Embodiment 3
[0241] Negative MIC data for indicating no antifungal effect
[0242] The test product is exposed to the Malassezia suspension. This mixture was deposited on the surface of the agar medium. The mixture was spread out and excess mixture was recovered prior to incubation. Cultivation was continued for at least 5 days at 30°C.
[0243] Products were placed in liquid modified Leeming and Notman medium (LNm) and tested in duplicate. The complete culture is 7.5 g / l or 150 g / l (concentrated form) based on dry matter.
[0244] Complete cultures were tested at 10% (for a concentration at 7.5 g / l) and 1% (for a concentration at 150 g / l).
[0245] The positive reference was 1% zinc pyrithione.
[0246] The solution of the product was concentrated twice to allow for dilution to 1 / 2 during contact with the Malassezia suspension.
[0247] Table 8
[0248]
[0249] M. globosa and M. restrictis strains were collected on agar slants and subcultured at 30° C. and grown until tested....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com