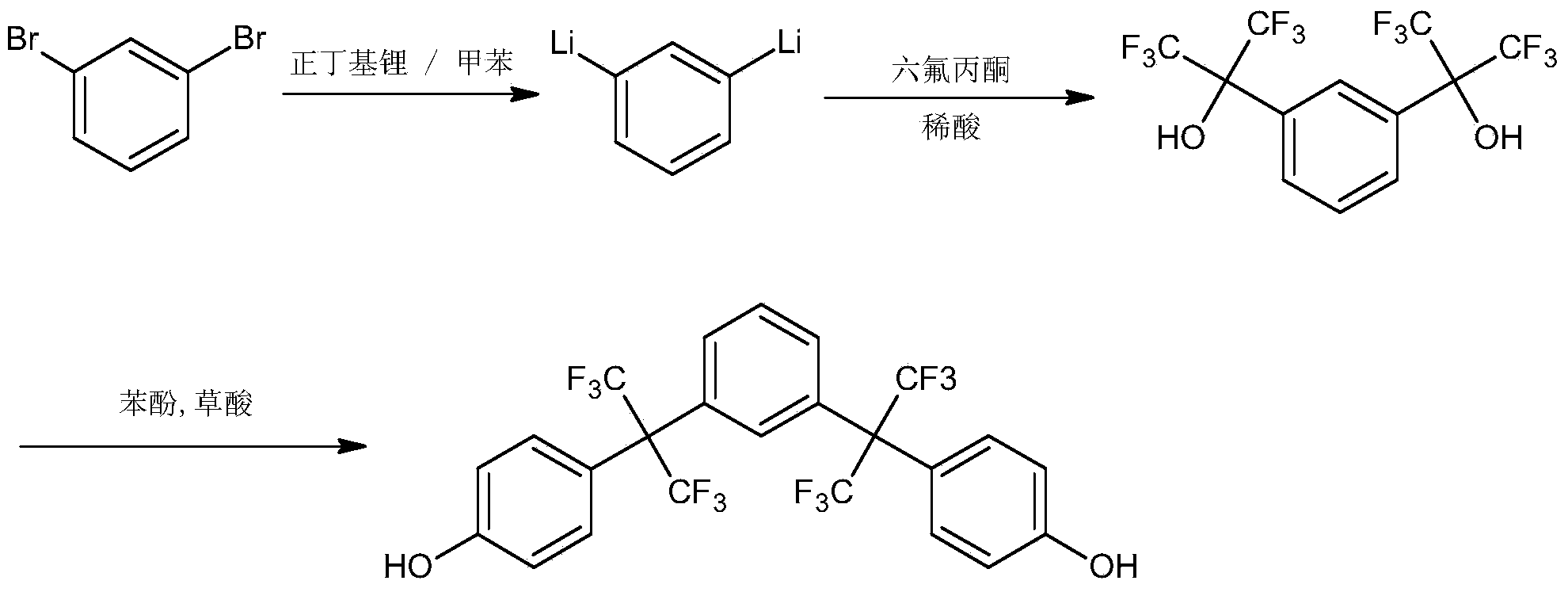
Preparation of novel fluorine-containing bisphenol compound
A technology of phenolic compounds and phenol, which is applied in the field of synthesis of novel fluorine-containing bisphenol compound 1,3-bis-hexafluoropropylidene)benzene, can solve the problems of few reports and no discovery of fluorine-containing phenol compounds, and achieve Low cost and wide application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Into a 500mL glass bottle equipped with a reflux condenser (the upper end of the condenser is equipped with a nitrogen protection balloon), a thermometer and an addition funnel, add 200ml of tetrahydrofuran and 2.8g (0.4mol) of lithium wire, heat to 40°C, and drop under vigorous stirring Add 11.8g (0.2mol) m-dibromobenzene, after the lithiation reaction is initiated, the temperature of the reaction solution rises to boiling quickly. Control the rate of addition to maintain a slight boiling state, and it takes about 1 hour for all the drops to be completed. Keep the slightly boiling state and continue to react for 2h, and the lithium filaments completely disappear.
[0020] Cool the above reaction solution to room temperature, add 62g (0.4mol) of hexafluoroacetone dropwise under stirring, control the rate of addition to keep the temperature of the reaction solution not higher than 40°C, complete the dropwise addition of hexafluoroacetone within 1h, and continue the natur...
Embodiment 2
[0024]Add 200ml of toluene-tetrahydrofuran (mass ratio 3:1) and 2.8g (0.4mol) of lithium wire into a 500mL glass bottle equipped with a reflux condenser (a nitrogen protection balloon is installed at the upper end of the condenser), a thermometer and an addition funnel, and heat At 60°C, 11.8 g (0.2 mol) of m-dibromobenzene was added dropwise under vigorous stirring. After the lithiation reaction was initiated, the temperature of the reaction solution rose to slightly boiling quickly. Control the rate of addition to maintain a slight boiling state, and it takes about 0.5 hours for all the drops to be completed (compared with the higher boiling point of tetrahydrofuran medium, the rate of addition can be faster). Keep the slightly boiling state and continue the reaction for 1h, and the lithium filaments completely disappear.
[0025] The above reaction solution was cooled to room temperature, and 62g (0.4mol) of hexafluoroacetone was added dropwise under stirring, and the rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com