Triterpenoid compounds and application thereof in diabetes treatment drugs
A compound and drug technology, applied in the field of biomedicine, can solve the problems of high adverse reactions and weak activity, and achieve the effect of good α-glucosidase inhibitory activity, small molecular weight, and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of embodiment 1 compound 1 and 2
[0048] 1. Preparation of Inonotus obliquus fermentation product by fermentation culture
[0049] 1), strains:
[0050] Inonotus obliquus is classified as Fuscoporia obliqua (Pers: Fr.) Aoshima, and is preserved by the China Agricultural Microorganism Culture Collection and Management Center, and the strain number is 1511C0001ACCC51184.
[0051] 2) Cultivation and preservation of seed strains
[0052]Solid medium: glucose 3g, potato extract powder 0.5g, KH2PO40.2g, soybean peptone 0.5g, agar 1.5g, add water to 100mL, adjust pH to 6.0.
[0053] Solid culture method: the strain is inoculated on a solid medium slant and cultured at 28°C for 7-10 days.
[0054] After the solid culture is over, place the slant at 4-10°C and refrigerate for later use.
[0055] 3) Shake flask seed culture
[0056] Medium: glucose 3g, yeast extract powder 1.0g, soybean peptone 1.0g, KH2PO 40.2g, add water to 100mL, adjust pH to 6.0. ...
Embodiment 2
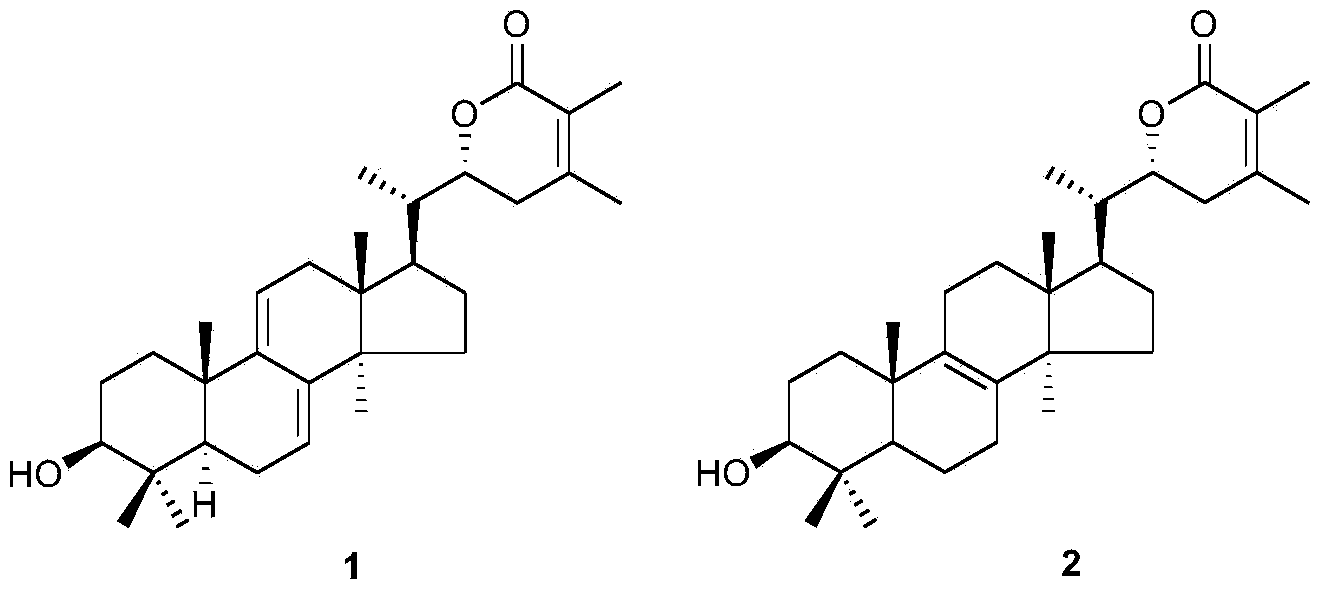
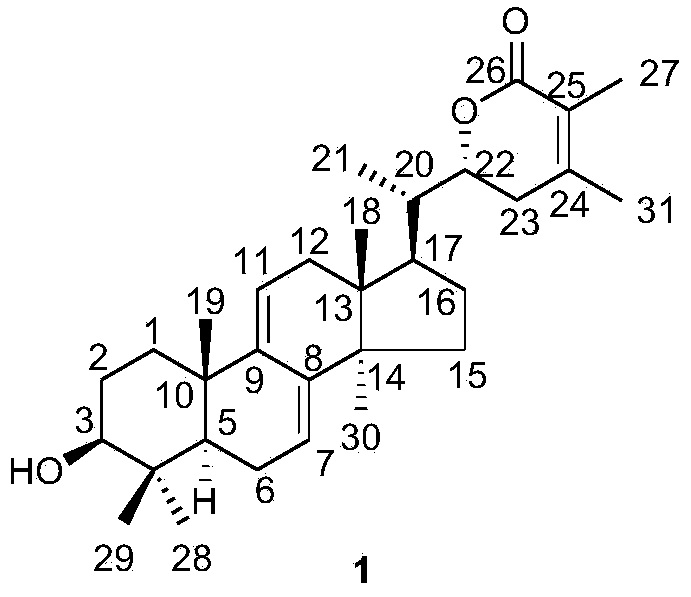
[0075] Embodiment 2: Physicochemical properties and spectral data of compound 1
[0076]
[0077] Compound 1: white amorphous powder; molecular formula is C 31 h 46 o 3 ;Optical rotation [α] 20 D +70.5(c0.088, CHCl 3 ); Infrared (KBr)ν max 3567, 2964, 2928, 1714, 1185, 1125cm -1 ; Hydrogen spectrum and carbon spectrum (CDCl 3 ,500MHz) See Table 1 Mass Spectrum (ESI) m / z: 467[M+H] + .
Embodiment 3
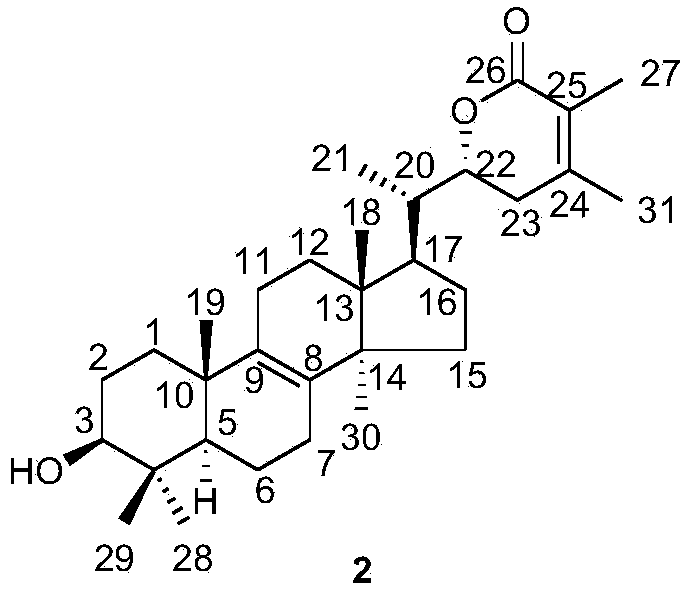
[0078] Embodiment 3: Physicochemical properties and spectral data of compound 2
[0079]
[0080] Compound 2: white amorphous powder; molecular formula is C 31 h 48 o 3 ;Optical rotation [α] 20 D +100.0(c0.19, CHCl 3 ); Infrared (KBr)ν max 3418,2934,1662,1595,1517,1458,1417,1189,1042,675cm -1 ; Hydrogen spectrum and carbon spectrum (CDCl 3 ,500MHz) See Table 1 Mass Spectrum (ESI) m / z: 469[M+H] + .
[0081] Table 1 NMR data of compounds 1 and 2
[0082]
[0083]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com