Method for forming nerve cells by induction and composition
A technology of composition and glial cells, which can be used in drug combinations, nervous system cells, nervous system diseases, etc., and can solve the problems of long cycle, many steps, and high failure rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
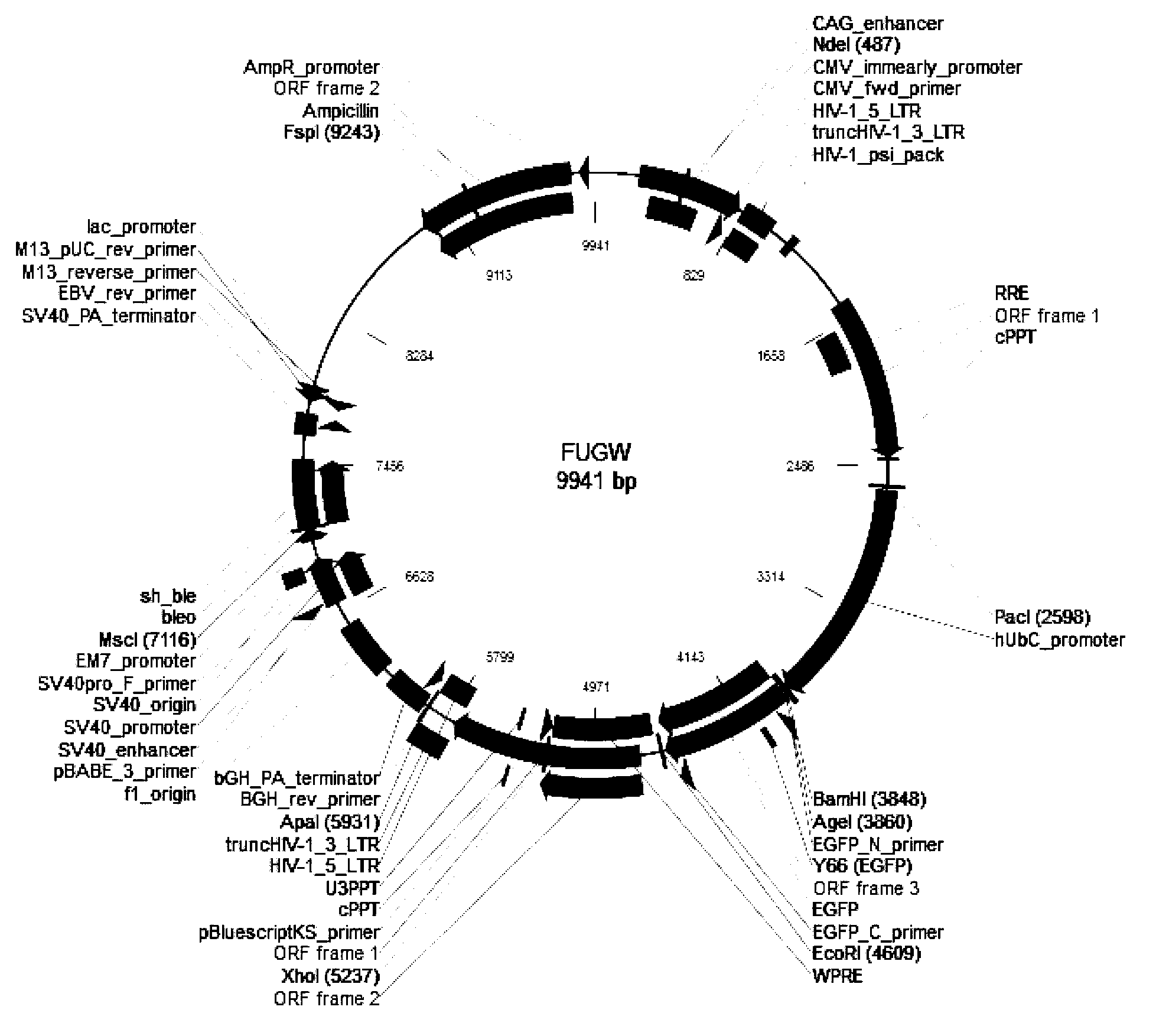
[0089] Construction of NeuroD1 lentiviral vector:
[0090] (1) Using the cloned NEUROD1 cDNA as a template, carry out a PCR reaction with amplification primers, and amplify to obtain a PCR product with a size of about 1.1 Kb.
[0091] (2) BamHI and AgeI double enzyme digestion is performed on the PCR product and the viral vector.
[0092] (3) T4 DNA ligase (purchased from Takara Company) was used to perform transformation reaction after ligation.
[0093] (4) Through the identification of transformants, positive clones were selected and sent for testing, and those whose sequencing sequence was consistent with the expected sequence were regarded as correct clones, and named as FUGW-NEUROD1 lentiviral vector.
Embodiment 2
[0095] Packaging of NeuroD1 lentiviral vector:
[0096] (1) Prepare DNA solutions of three plasmids in the lentiviral packaging system (FUGW-NEUROD1 lentiviral vector 20 μg, pCMV-dR8.2 dvpr vector (purchased from Addgene) 15 μg, pCMV-VSV-G vector (purchased from Addgene) 10 μg , mixed with the corresponding volume of Opti-MEM to evenly dilute, adjust the total volume to 2.5ml, and incubate at room temperature for 5 minutes.
[0097] (2) 100 μl of Lipofectamine2000 (purchased from invitrogen) reagent was mixed and diluted with 2.4 ml of Opti-MEM (purchased from invitrogen) in another tube, and incubated at room temperature for 5 minutes.
[0098] (3) Mix the diluted DNA described in (1) with the diluted Lipofectamine2000 described in (2), and gently invert and mix within 5 minutes. Incubate at room temperature for 20 min.
[0099](4) Transfer the mixture of DNA and Lipofectamine 2000 to the culture medium of 293T cells, mix well, and store at 37°C, 5% CO 2 cultured in a cell...
Embodiment 3
[0104] NeuroD1 lentivirus induced glial cells;
[0105] (1) When the glial cell U87 density reached 50%-60%, virus infection was carried out, and the cell culture medium was replaced with serum-free medium before infection.
[0106] (2) According to the MOI value of U87 cells (MOI=5), add an appropriate amount of NEUROD1 lentivirus (number of viruses added = number of cells x MOI value). Observe the cell state after 12 hours: if there is no obvious cytotoxic effect, replace the medium after continuing to culture for 24 hours; if there is obvious cytotoxic effect, replace the medium immediately.
[0107] (3) Medium replacement: Three days after U87 cells were infected with NEUROD1 lentivirus, the original medium was gradually replaced with Neurobasal medium (Invitrogen, 12348-017) containing factor N27 (Invitrogen, 17504-044,) . The replacement method is to keep 1 / 2 of the original medium each time, add 1 / 2 of the fresh Neurobasal medium with N27 factor, and replace the mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com