Amide derivative containing double-bond gramine and preparation method and application thereof
A technology of amides and derivatives, which is applied in the field of amides derivatives containing double bond anophylline and its preparation, which can solve the problems of poor compounding performance, fast release rate, and reduced antifouling effect, so as to prevent adhesion , less environmental hazards, simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
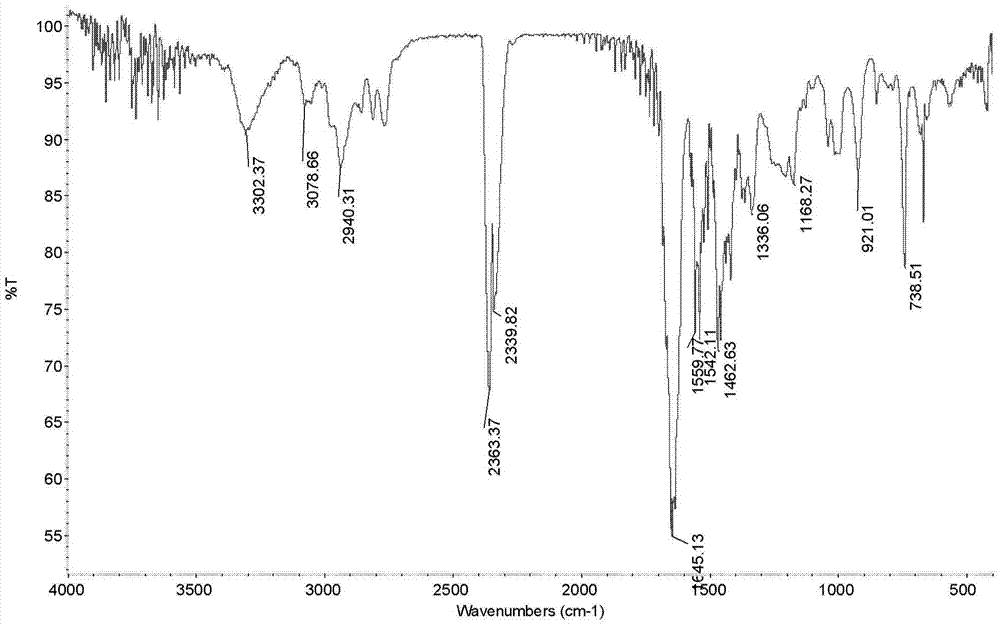
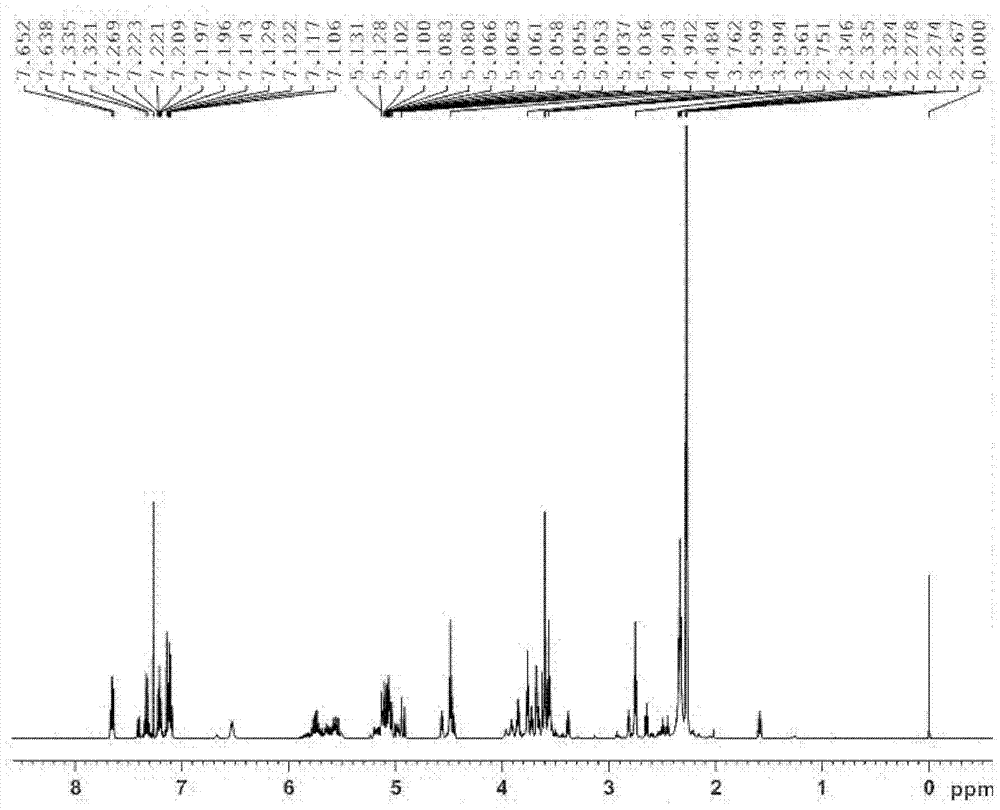
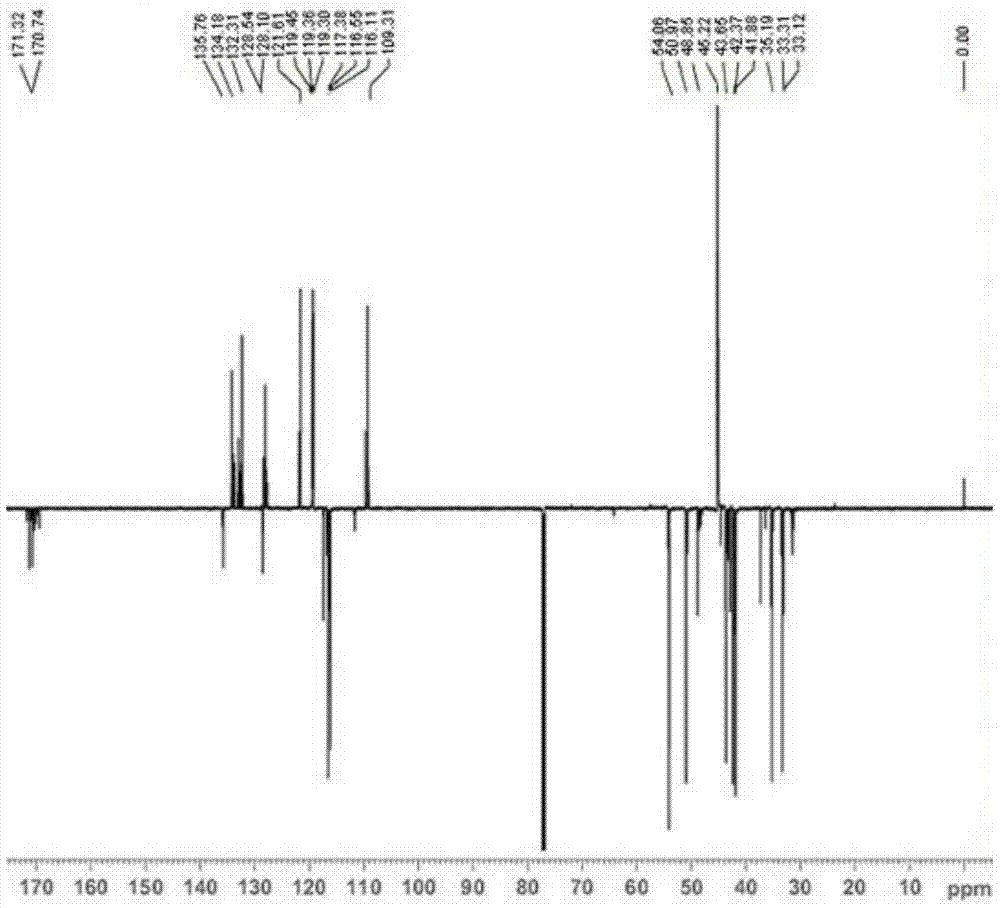
[0047] Example 1: Preparation of N-allyl-3-(3-((dimethylamino)methyl)-1-indolyl)propionamide
[0048] 1. Preparation of N,N'-diallyldithiodipropionamide
[0049] Add 17.1g (0.3mol) allylamine and 2.5ml triethylamine in sequence to a 250mL three-necked flask equipped with a stirring device, a condenser, and a thermometer, and control the reaction temperature to 0°C through an ice-water bath. 23.8g (0.1mol) dimethyl β-dithiodipropionate was added dropwise within 2.5h. After the dropwise addition was completed, the ice-water bath was removed, and the reaction was continued at room temperature for 48 h. The reaction was terminated, and a yellow solid product was obtained by suction filtration. After being washed twice with water, dried, and recrystallized with ethanol, 25.5 g of white flaky crystals were obtained with a yield of 88.4% and a melting point of 135.7-137.2°C.
[0050] 2. Preparation of N-allyl-3-(1-indolyl)propionamide
[0051] Weigh 5.8g (0.05mol) of indole and 15...
Embodiment 2
[0057] Example 2: Preparation of N-allyl-3-(3-((dimethylamino)methyl)-5-bromo-1-indolyl)propionamide
[0058] 1. Preparation of N,N'-diallyldithiodipropionamide
[0059] The preparation method is the same as in Example 1.
[0060] 2. Preparation of N-allyl-3-(5-bromo-1-indolyl)propionamide
[0061] Weigh 9.8g (0.05mol) of 5-bromoindole and 15.1g (0.05mol) of N,N’-diallyldithiodipropionamide respectively and dissolve them in 50mL of anhydrous THF. Add 50mL of anhydrous THF and 3.6g (0.09mol) NaH (60% content) in sequence into a 250mL three-necked flask equipped with a stirring device and a thermometer, and add the THF solution of indole to the above system dropwise at 0°C. After dropping, continue to react for 30 minutes, then add the THF solution of N,N'-diallyldithiodipropionamide dropwise to the above system at 0°C. After dropping, the reaction was continued for 5 hours at room temperature. After the reaction, add 50mL of saturated NH 4 Cl solution, let it stand, take t...
Embodiment 3
[0066] Example 3: Preparation of N-allyl-3-(3-((dimethylamino)methyl)-5,6-dichloro-1-indolyl)propionamide
[0067] 1. Preparation of N,N'-diallyldithiodipropionamide
[0068] The preparation method is the same as in Example 1.
[0069] 2. Preparation of N-allyl-3-(5,6-dichloro-1-indolyl)propionamide
[0070] Weigh 9.3g (0.05mol) of 5,6-dichloroindole and 15.1g (0.05mol) of N,N’-diallyldithiodipropionamide respectively and dissolve them in 50mL of anhydrous THF. Add 50mL of anhydrous THF and 3.6g (0.09mol) NaH (60% content) in sequence into a 250mL three-necked flask equipped with a stirring device and a thermometer, and add the THF solution of indole to the above system dropwise at 0°C. After dropping, continue to react for 30 minutes, then add the THF solution of N,N'-diallyldithiodipropionamide dropwise to the above system at 0°C. After dropping, the reaction was continued for 5 hours at room temperature. After the reaction, add 50mL of saturated NH 4 Cl solution, let i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com