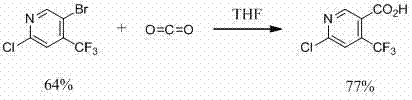
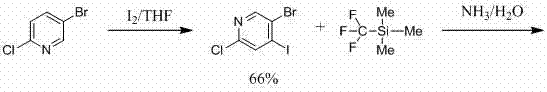
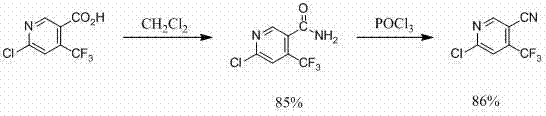
Method for preparing 6-chlorine-4-trifluoromethyl-3-cyanopyridine
A technology of trifluoromethyl and cyanopyridine, applied in the field of chemistry, can solve the problems of long process steps, harsh operating conditions, complicated operations, etc., and achieves the effects of low reaction cost, short reaction steps, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine, comprising the steps of:
[0038] (1) 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine ( )Synthesis
[0039] Dissolve 90 g of 2,6-dichloro-4-trifluoromethyl-3-cyanopyridine in 450 g of methanol, add 279 g of sodium methoxide, and react at room temperature for 220 minutes. The reaction is complete as detected by thin-layer chromatography. Spin to dry methanol, add 400g of distilled water was extracted twice with 200g ethyl acetate, and the organic phase was taken and washed with anhydrous Na 2 SO 4 Dry for 2 hours, filter, and spin-dry to obtain 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine (78.3 g) as an oil, with a yield of 88.6%.
[0040] (2) Synthesis of 6-methoxy-4-trifluoromethyl-3-cyanopyridine (Ⅲ)
[0041] Dissolve 80 g of the obtained 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine in 400 g of methanol, add 8 g of palladium carbon, control the pressure at 1.5 MPa, replace hy...
Embodiment 2
[0049] A preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine, comprising the steps of:
[0050] (1) 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine ( )Synthesis
[0051] Dissolve 90 g of 2,6-dichloro-4-trifluoromethyl-3-cyanopyridine in 500 g of methanol, add 360 g of sodium methoxide, and react at room temperature for 200 minutes. It is detected by thin-layer chromatography that the reaction is complete, spin dry methanol, and add 450g of distilled water, extracted twice with 250g of ethyl acetate, take the organic phase, and use anhydrous Na 2 SO 4 Dried for 2 hours, filtered, and spin-dried to obtain oily 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine (78.9 g), with a yield of 89.3%.
[0052] (2) Synthesis of 6-methoxy-4-trifluoromethyl-3-cyanopyridine (Ⅲ)
[0053] Dissolve 80 g of the obtained 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine in 620 g of methanol, add 8 g of platinum oxide, control the pressure at 1.8 MPa, replace hydrogen f...
Embodiment 3
[0061] A preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine, comprising the steps of:
[0062] (1) 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine ( )Synthesis
[0063] Dissolve 90 g of 2,6-dichloro-4-trifluoromethyl-3-cyanopyridine in 600 g of methanol, add 405 g of sodium methoxide, and react at room temperature for 240 minutes. It is detected by thin-layer chromatography that the reaction is complete, spin dry methanol, and add 500g of distilled water, extracted twice with 250g of ethyl acetate, take the organic phase, and use anhydrous Na 2 SO 4 Dry for 2 hours, filter and spin-dry to obtain 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine (80.57 g) as an oil, with a yield of 91.2%.
[0064] (2) Synthesis of 6-methoxy-4-trifluoromethyl-3-cyanopyridine (Ⅲ)
[0065] Dissolve 80 g of the obtained 2-chloro-6-methoxy-4-trifluoromethyl-3-cyanopyridine in 620 g of methanol, add 8 g of palladium carbon, control the pressure at 1.8 MPa, replace hydrogen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com