A cost-effictive method for expression and purification of recombinant proteins in plants
A plant and protein technology, applied in peptide preparation methods, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problem of low expression level, complex expression and purification of ELP-intein fusion, and difficulty in large-scale recovery And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Purification of Recombinant Antitumor Proteins from Transgenic Rice Seeds
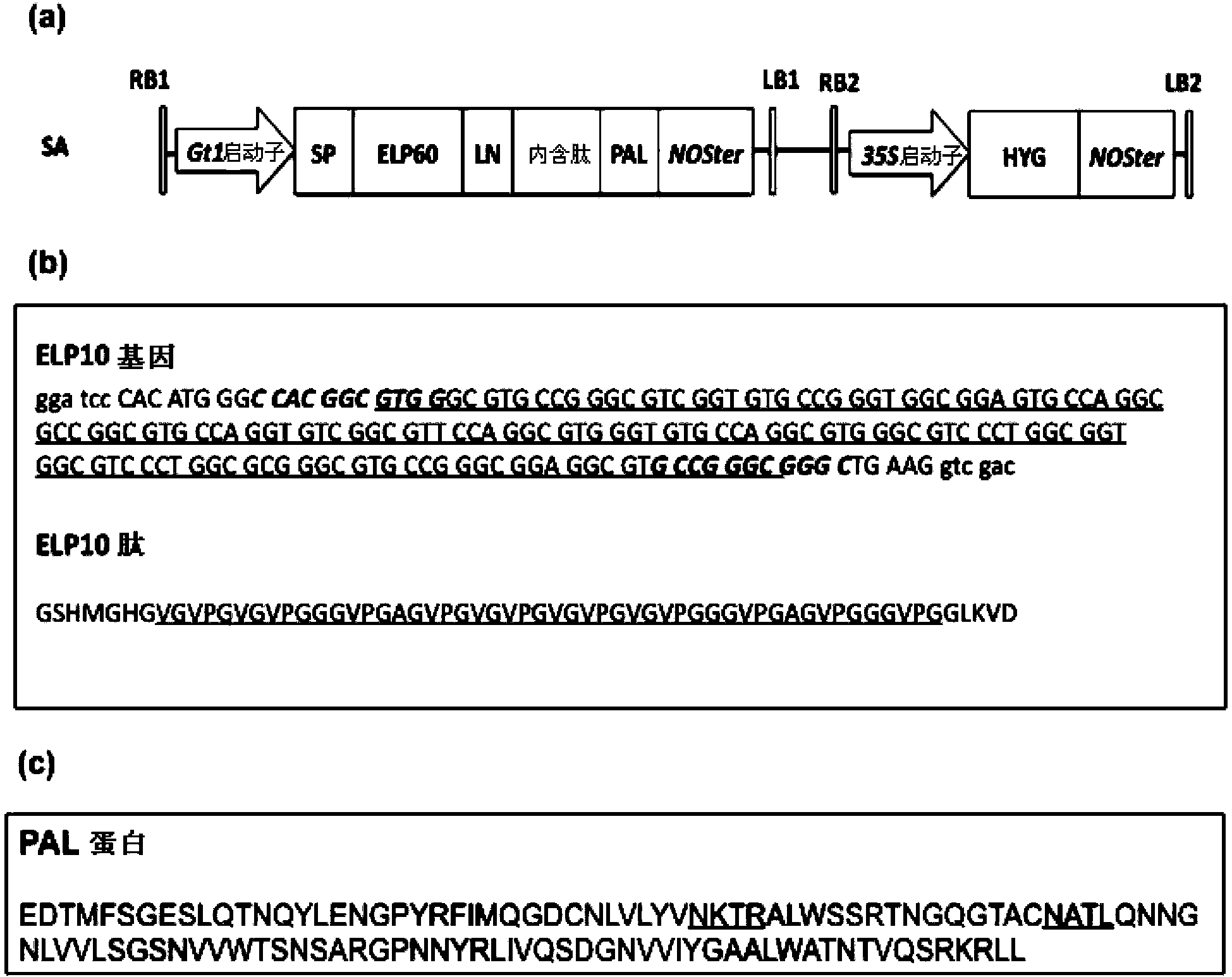
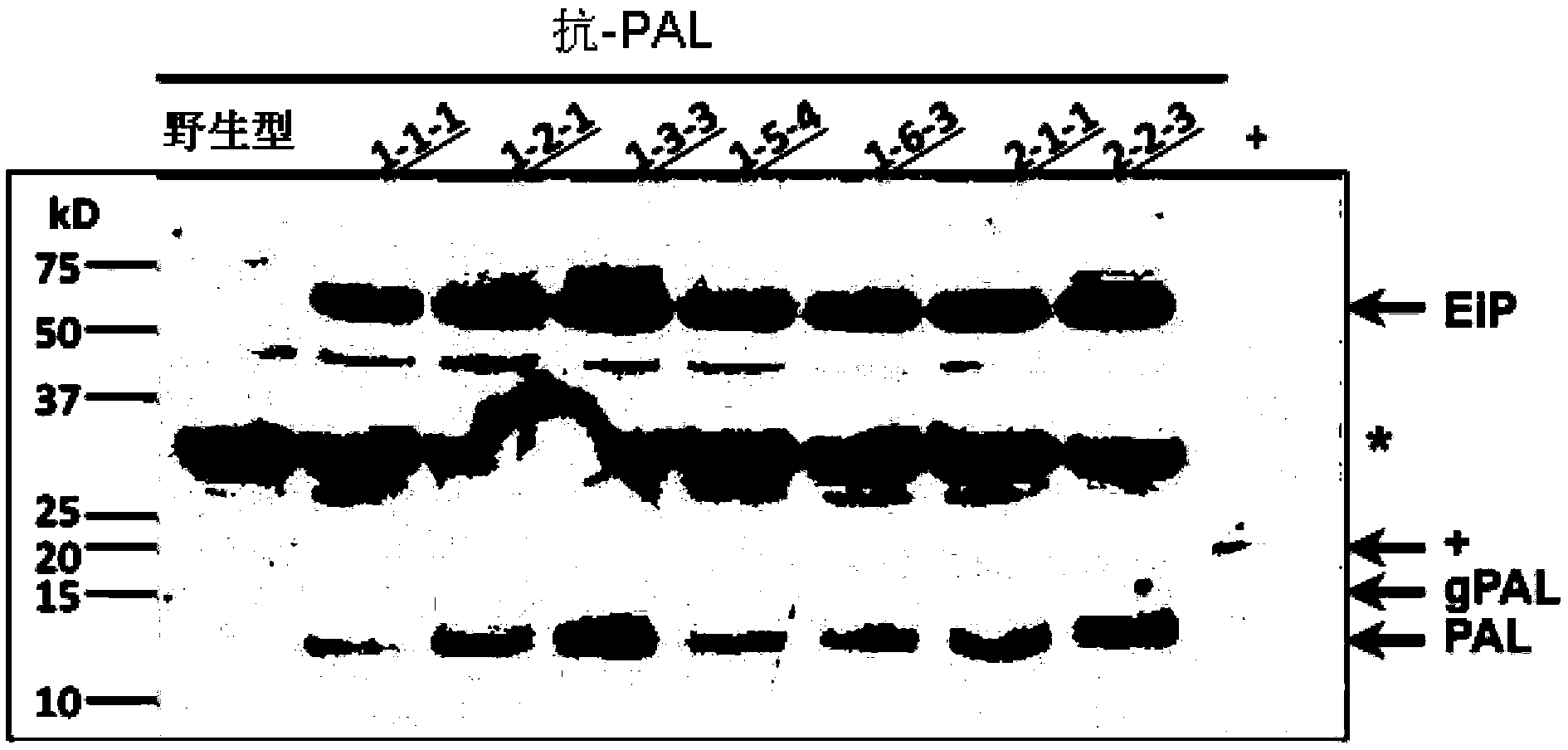
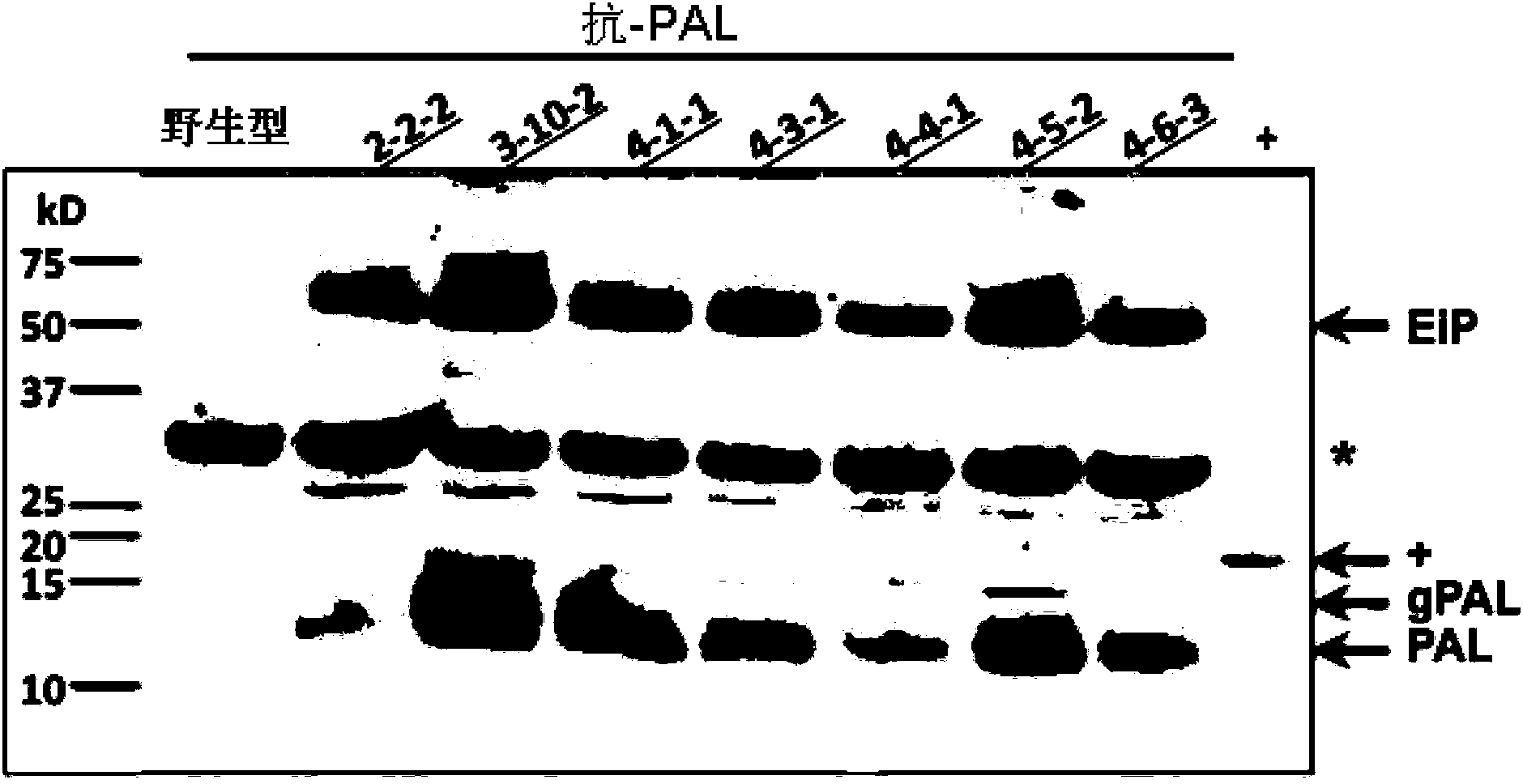
[0067] In this example, transgenic rice seeds were used as the production system, and the molecular weight (Mw) was about 12.5kD (the protein sequence is shown in figure 1 (c) Pandanus lectin (PAL) as the target protein. PAL shows inhibitory activity against the proliferation of several human cancer cell lines (HepG2, A549, DLD-1, U87 and Capan-2). PAL is expressed as a fusion of an ELP-intein tag, wherein ELP comprises 60 repeats of the "VPGXG" peptide, and the intein protein is cleaved at its C-terminus in response to low pH. The ELP-intein tag is inserted at the N-terminus of PAL, so that after purification of the ELP-intein-PAL fusion protein from rice seeds, intein cleavage can be triggered by lowering the pH of the buffer to isolate the target PAL protein and Ei tag.
[0068] The expression cassette of the ELP-intein-PAL fusion protein is shown in figure 1 (a), the expression casse...
Embodiment 2
[0082] Purification of recombinant human G-CSF from transgenic tobacco
[0083] In this embodiment, transgenic tobacco leaves were used as the production system, while human granulocyte colony-stimulating factor (hG-CSF), an important human cytokine that has been widely used in tumors, was used as the target protein. medicine and infection protection.
[0084] The hG-CSF fusion expression chimera was constructed using the CaMV35S promoter in the binary vector pBI121, and a phaseolin signal peptide was introduced to direct the expressed fusion protein into the secretory pathway of plant cells. The expression cassette is shown in Image 6 . Ubiquitin is introduced to improve the expression of the target protein and will be accurately processed from the fusion protein by endogenous ubiquitin-specific proteases (Ubp) (Tian, L. and Sun, S.S.M., BMC Biotechnol (2011) 11:91). Image 6 The sequence encoding the hG-CSF-intein2-ELP110 fusion protein indicated by brackets is shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com