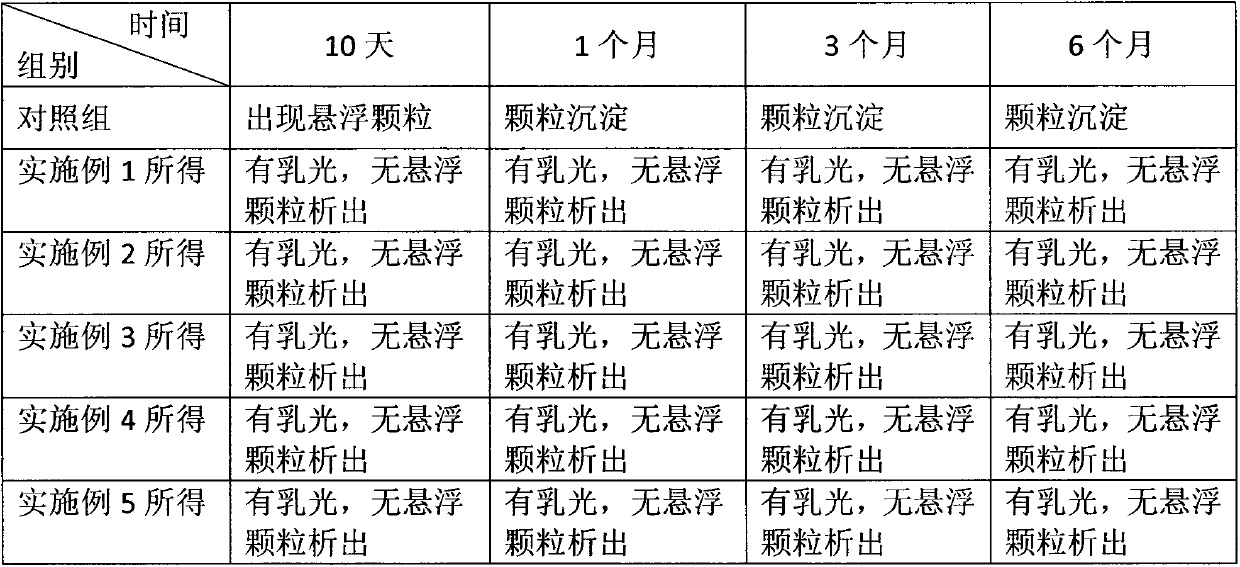
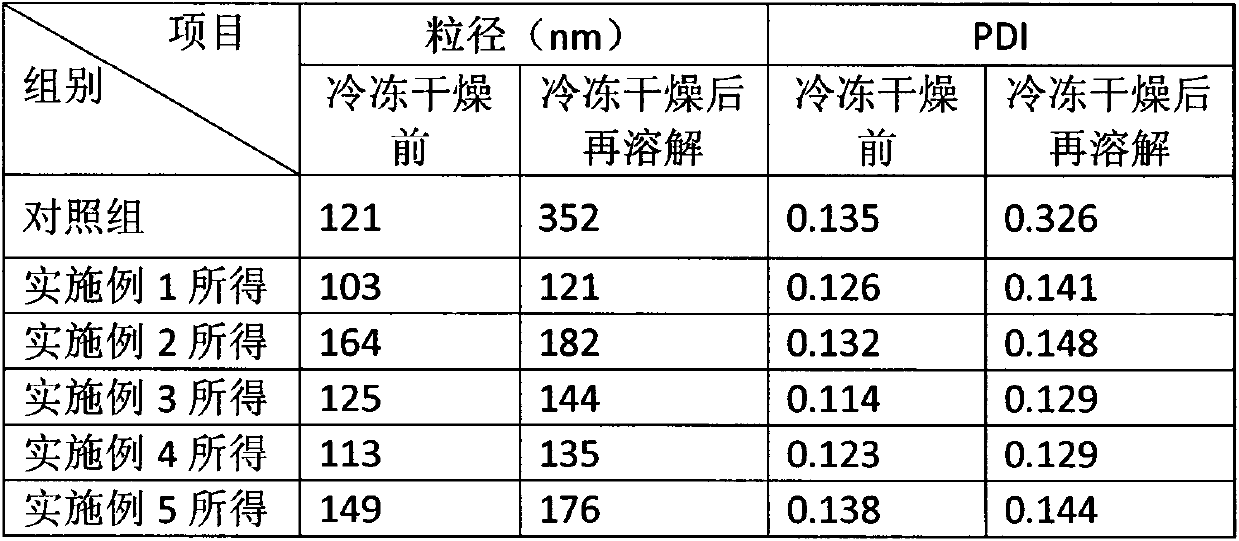
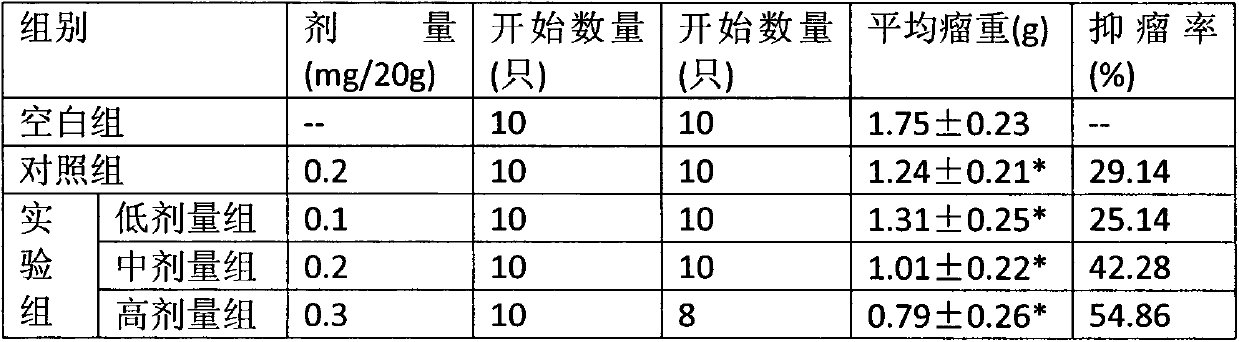
Polyethylene glycol vitamin E succinate and calprotectin modified nanoparticle and preparation method thereof
A technology of polyethylene glycol and succinate, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve limitations, small molecular weight of phospholipids, and insufficient stability of nano-formulations, etc. problem, to achieve the effect of enhanced targeting, improved stability, small changes in particle size and polydispersity coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Prescription: 1mg betulinic acid, 20mg soybean lecithin, 2mg sophorolipid, 1mg calreticulin, 5mg polyethylene glycol vitamin E succinate.
[0020] Preparation Process:
[0021] (1) dissolve betulinic acid, soybean lecithin and sophorolipid with 10ml ethanol;
[0022] (2) Ethanol was removed by vacuum rotary evaporation, and vacuum-dried for 4 hours to obtain a dry film;
[0023] (3) Dissolve polyethylene glycol vitamin E succinate and calprotectin in 10 ml of pure water, and add them to the dry film to fully dissolve the film;
[0024] (4) Filtrate through a 0.22 μm microporous membrane to obtain the product.
Embodiment 2
[0026] Prescription: 1mg morin, 40mg soybean lecithin, 4mg sophorolipids, 2mg calreticulin, 10mg polyethylene glycol vitamin E succinate.
[0027] Preparation Process:
[0028] (1) dissolve morin, soybean lecithin and sophorolipid with 10ml ethanol;
[0029] (2) Ethanol was removed by vacuum rotary evaporation, and vacuum-dried for 8 hours to obtain a dry film;
[0030] (3) Dissolve polyethylene glycol vitamin E succinate and calprotectin in 10 ml of pure water, and add them to the dry film to fully dissolve the film;
[0031] (4) Filtrate through a 0.22 μm microporous membrane to obtain the product.
Embodiment 3
[0033] Prescription: 1mg paclitaxel, 30mg egg yolk lecithin, 3mg sophorolipids, 1.5mg calreticulin, 8mg polyethylene glycol vitamin E succinate.
[0034] Preparation Process:
[0035] (1) Mix paclitaxel, egg yolk lecithin and sophorolipid, add 10ml ethanol to dissolve;
[0036] (2) Ethanol was removed by vacuum rotary evaporation, and vacuum-dried for 6 hours to obtain a dry film;
[0037] (3) Dissolve polyethylene glycol vitamin E succinate and calprotectin in 10 ml of pure water, and add them to the dry film to fully dissolve the film;
[0038] (4) Filtrate through a 0.22 μm microporous membrane to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com