Z-selective ring-closing metathesis reactions
A reaction and selection technology, applied in the field of Z-selective closed-loop metathesis reaction, which can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] 1. Overview of Certain Embodiments of the Invention
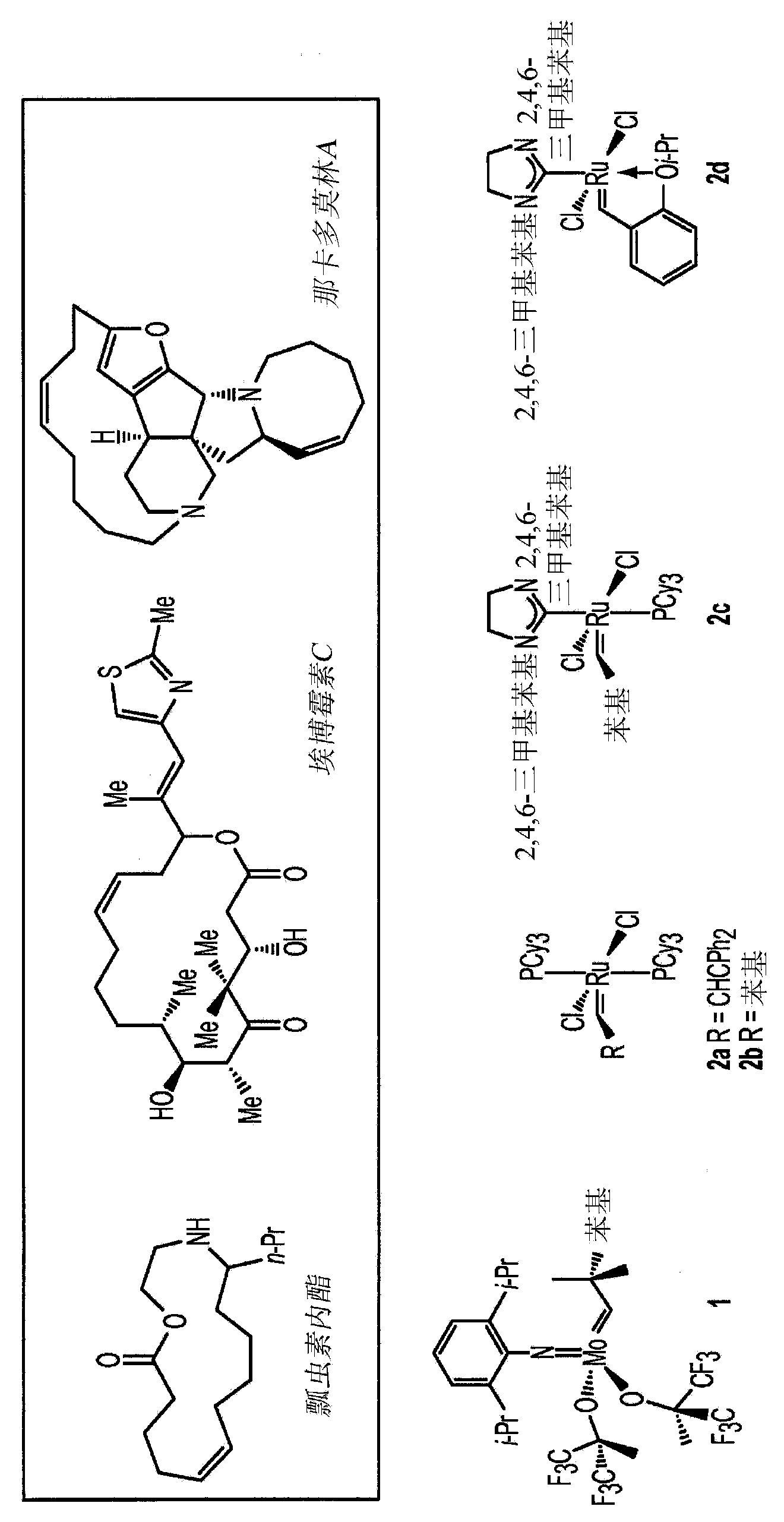
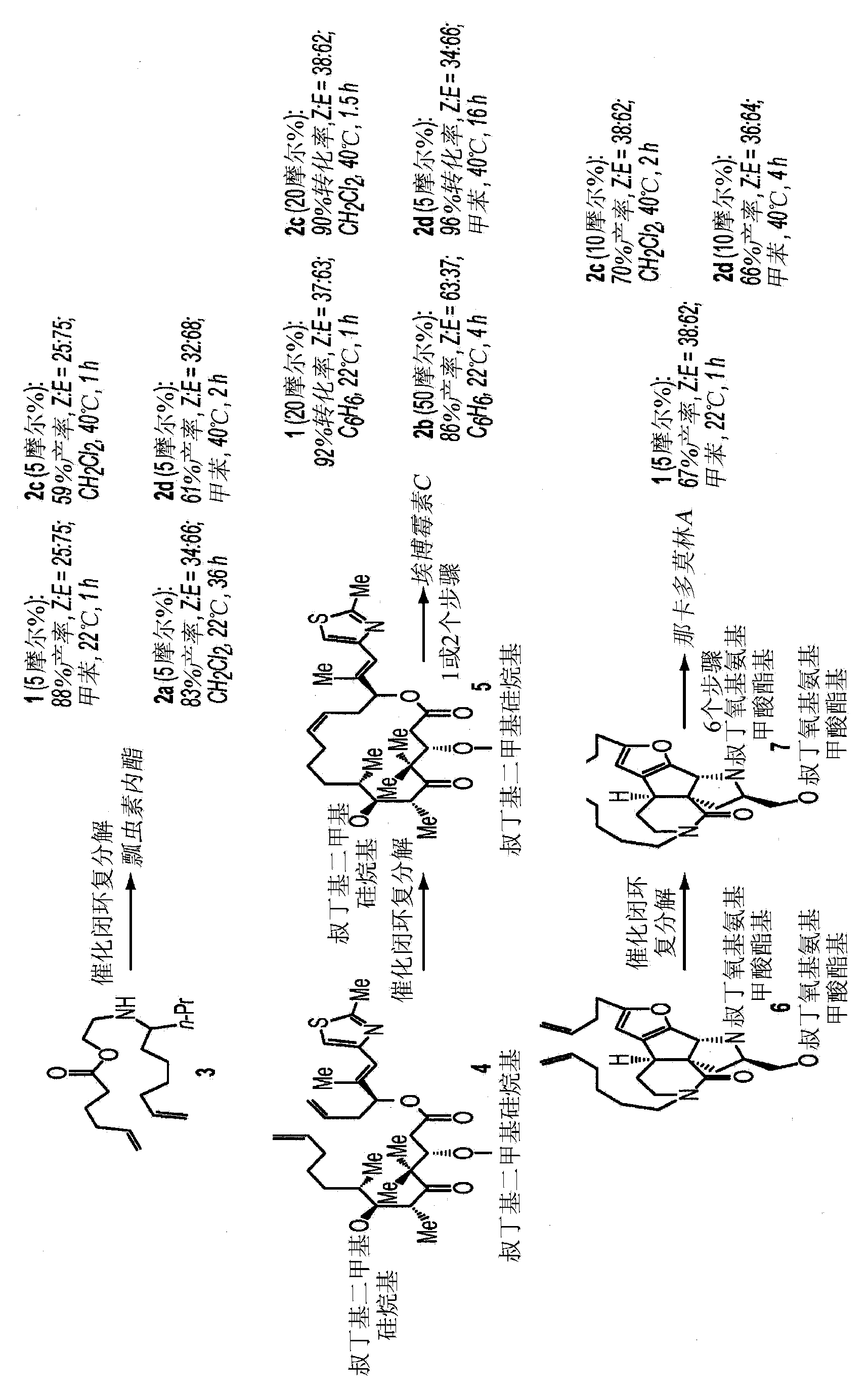
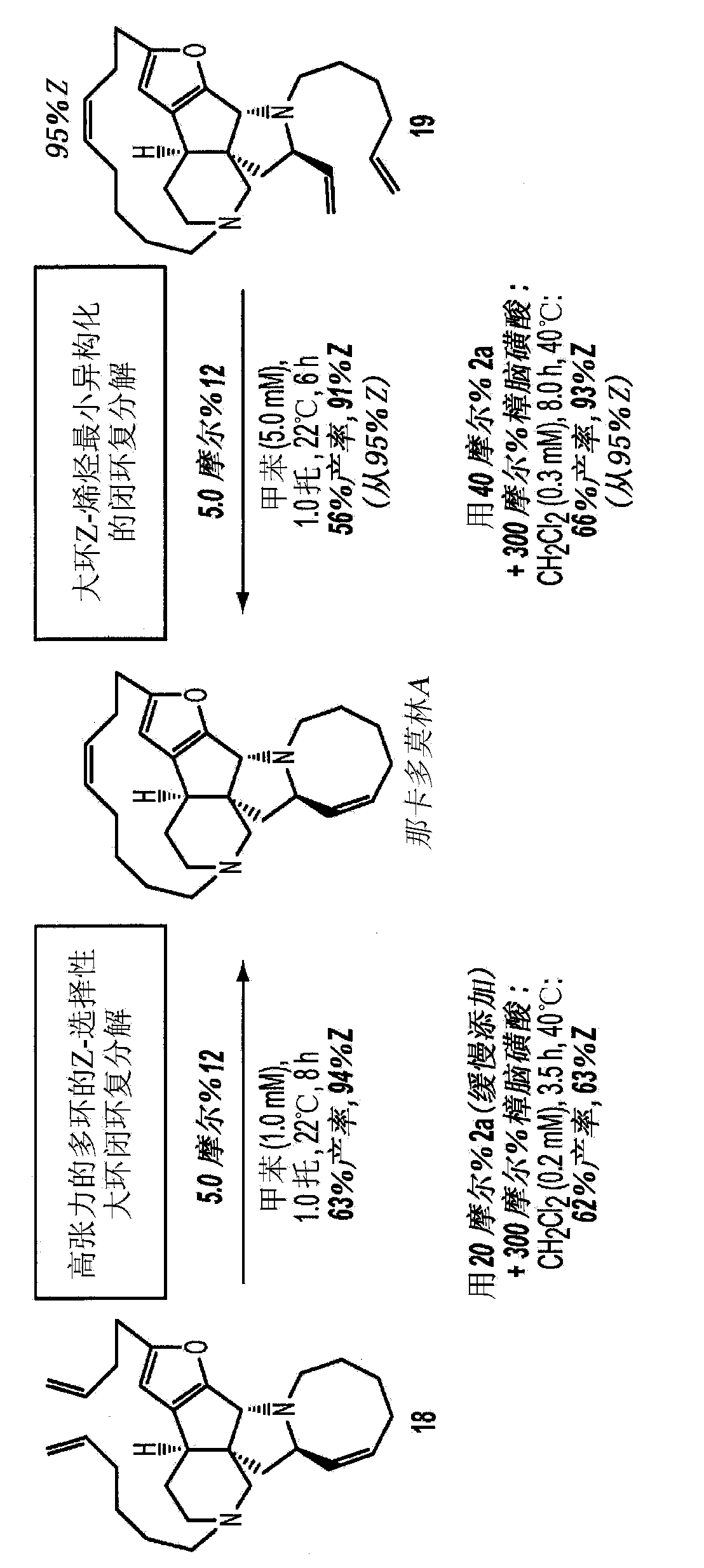
[0018] The present invention provides methods for the synthesis of macrocyclic olefins by catalytic RCM reactions, which proceed with high efficiency and stereoselectivity, in some embodiments, up to 97% Z isotropic Construct. Utility was demonstrated by application to the preparation of several bioactive molecules, including the anticancer epothilone A and the antimicrobial nakadomulin A, the synthesis of which had been disrupted by late-stage non-selective RCM. It is demonstrated herein that complexes derived from tungsten or molybdenum provide high efficiency and stereoselectivity, even at relatively high concentrations. The reaction proceeds via the desired pathway rather than the undesired alternative: efficient RCM and cross-metathesis between the incidentally formed self-coupling product and ethylene to regenerate the substrate, but minimal isomerization of macrocyclic Z-olefins.
[0019] Unexpected discover...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com