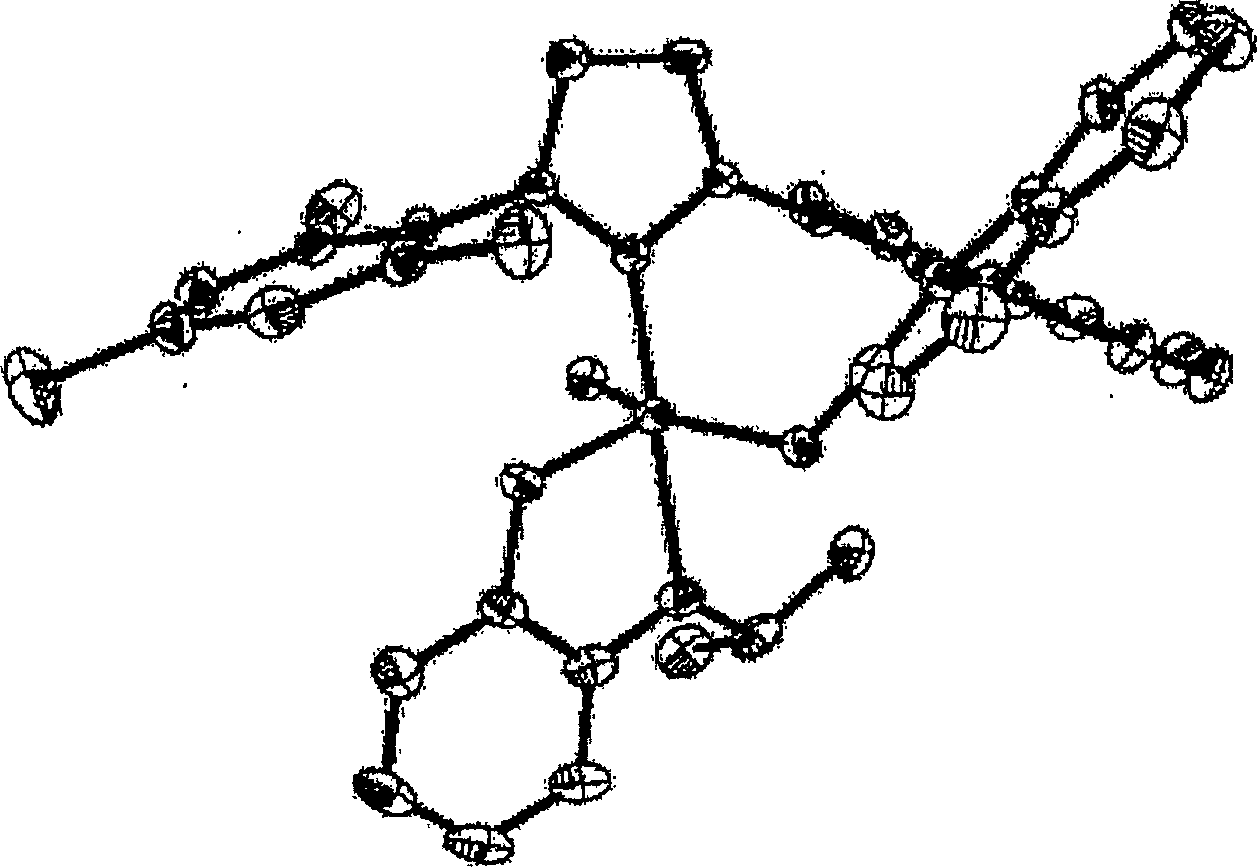
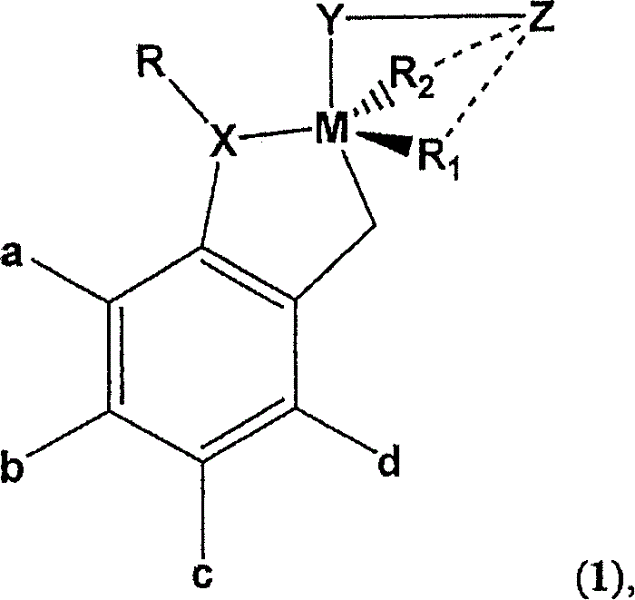
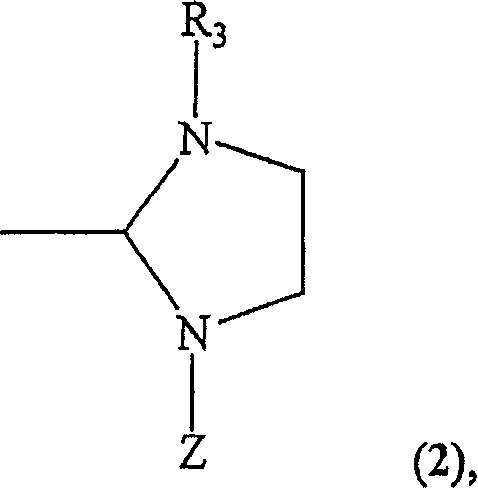
Recyclable chiral metathesis catalysts
A metal catalyst, a chiral technology, applied in the preparation of the catalyst, a chiral transition metal catalyst for a stereoselective olefin metathesis reaction, the preparation of the chiral catalyst, a chiral transition metal catalyst, a catalytic stereoselective olefin metathesis reaction, transition In the field of metal catalysts, it can solve problems such as instability and limited applicability, and achieve the effect of easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 1-Bromo-2-methoxynaphthalene. Prepared according to literature methods. Majetich, G.; Hicks, R.; Reister, S., J. Org. Chem. (1997), 62, 4321-4326.
[0063]IR(NaCl): 3047(w), 2972(m), 2943(m), 2843(m), 1622(s), 1596(m), 1501(s), 1467(m), 1454(m), 14.40(w), 1353(m), 1335(m), 1271(s), 1247(w), 1219(m), 1187(m), 1173(w), 1153(w), 1064(s), 1023(m), 892(m), 840(m), 813(m), 803(s), 763(m), 743(s), 518(m).
[0064] 1 H NMR (400MHz, CDCl 3 ): δ8.19(d, J=8.6Hz, 1H), 7.75(t, J=8.5Hz, 2H), 7.53(t, J=8.2Hz, 1H), 7.36(t, J=7.9Hz, 1H ), 7.22 (d, J=9.0Hz, 1H), 3.98 (s, 3H).
[0065] 13 C NMR (100MHz, CDCl 3 ): δ153.88, 133.25, 130.94, 129.09, 128.16, 127.86, 126.24, 124.43, 113.74, 108.78, 57.17.
[0066] HRMS calculated value C 11 h 9 BrO: 235.9837. Found: 235.9838.
[0067] Analytical calculated value C 11 h 9 BrO: C, 55.72; H, 3.83. Found: C, 55.66; H, 3.82.
Embodiment 2
[0069] Methyl 1-methoxynaphthoate. Prepared according to the method described in the literature Hattori, T.; Hotta, H.; Miyano, S. Bull. Chem. Soc. Jpn. (1993), 66, 613-622.
[0070] IR(NaCl): 3058(w), 2997(w), 2948(m), 2846(w), 1725(s), 1628(m), 1597(m), 1570(m), 1504(m), 1466(m), 1445(m), 1434(m), 1373(s), 1343(s), 1279(s), 1239(s), 1214(m), 1191(m), 1153(m), 1133(s), 1084(s), 1001(m), 829(m), 802(m), 787(m), 768(s), 715(w).
[0071] 1 H NMR (400MHz, CDCl 3 ): δ8.30-8.26(m, 1H), 7.87-7.84(m, 2H), 7.62(d, J=8.6Hz, 1H), 7.61-7.54(m, 2H), 4.07(s, 3H), 3.99 (s, 3H).
[0072] 13 C NMR (100MHz, CDCl 3 ): δ166.85, 158.44, 136.93, 128.47, 128.02, 126.83, 126.68, 123.78, 123.77, 119.34, 63.56, 52.41.
[0073] HRMS calculated value C 13 h 12 o 3 : 216.0786. Measured value: 216.0787.
[0074] Analytical calculated value C 13 h 12 o 3 : C, 72.21; H, 5.59. Found: C, 72.50; H, 5.52.
Embodiment 3
[0076] 1-(-)-menthyloxy-2-naphthoic acid-(-)-menthyl ester is described in Hattori, T.; Hotta, H.; Miyano, S. Bull. Chem. Soc. Jpn. (1993) , 66, prepared by the method of 613-622.
[0077] IR(NaCl): 3058(w), 2954(s), 2956(s), 2869(m), 1719(s), 1625(w), 1598(w), 1568(w), 1502(w), 1457(m), 1387(m), 1371(m), 1342(m), 1319(m), 1277(s), 1235(s), 1215(m), 1203(m), 1181(w), 1148(s), 1137(s), 1098(m), 1081(s), 1038(w), 1007(w), 983(m), 962(m), 920(w), 823(w), 801(w), 766(m), 741(w).
[0078] 1 H NMR (400MHz, CDCl 3 ): δ8.32(d, J=8.5Hz, 1H), 7.81(d, J=7.7Hz, 1H), 7.67(d, J=8.6Hz, 1H), 7.56-7.48(m, 3H), 5.04 (td, J=10.9, 4.4Hz, 1H), 4.34(td, J=10.9, 4.2Hz, 1H), 2.67(quintetd, J=6.8, 2.2Hz, 1H), 2.21-2.16(m, 1H), 2.05(quintetd, J=7.2, 2.7Hz, 1H), 1.79-1.52(m, 7H), 1.28-0.83(m, 23H), 0.74(d, J=6.6Hz, 3H).
[0079] 13 C NMR (100MHz, CDCl 3 ):δ166.77,154.10,136.24,130.12,127.86,127.74,126.22,126.00,124.56,122.40,121.15,82.11,74.59,49.64,47.27,41.17,39.97,34.59,34.46,31.67,31.61,26....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com