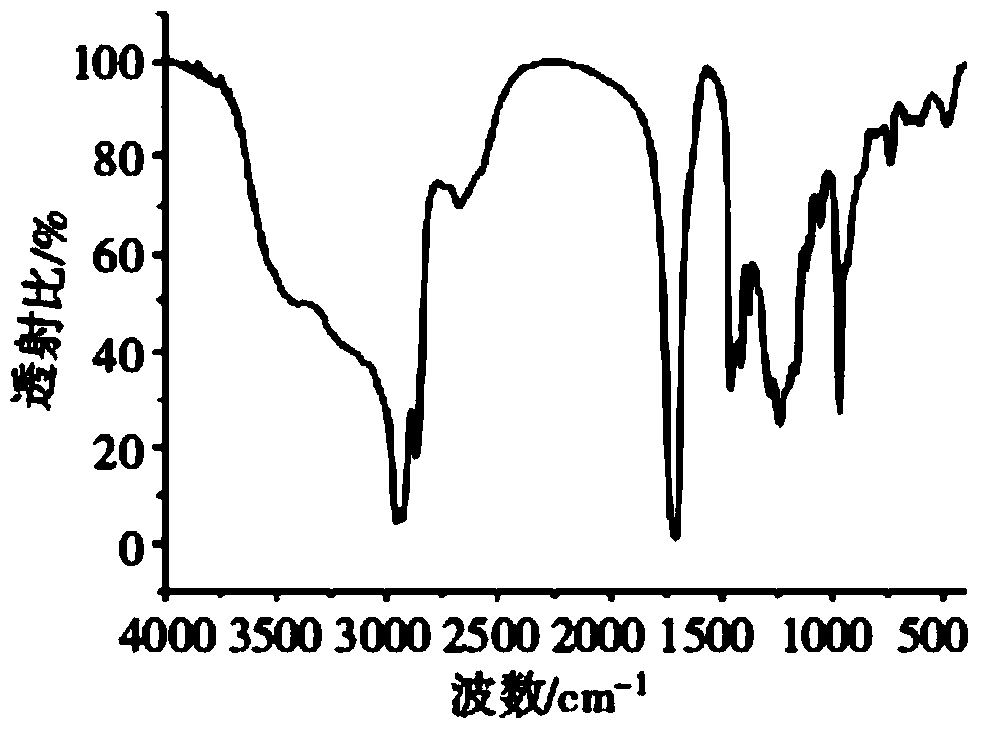
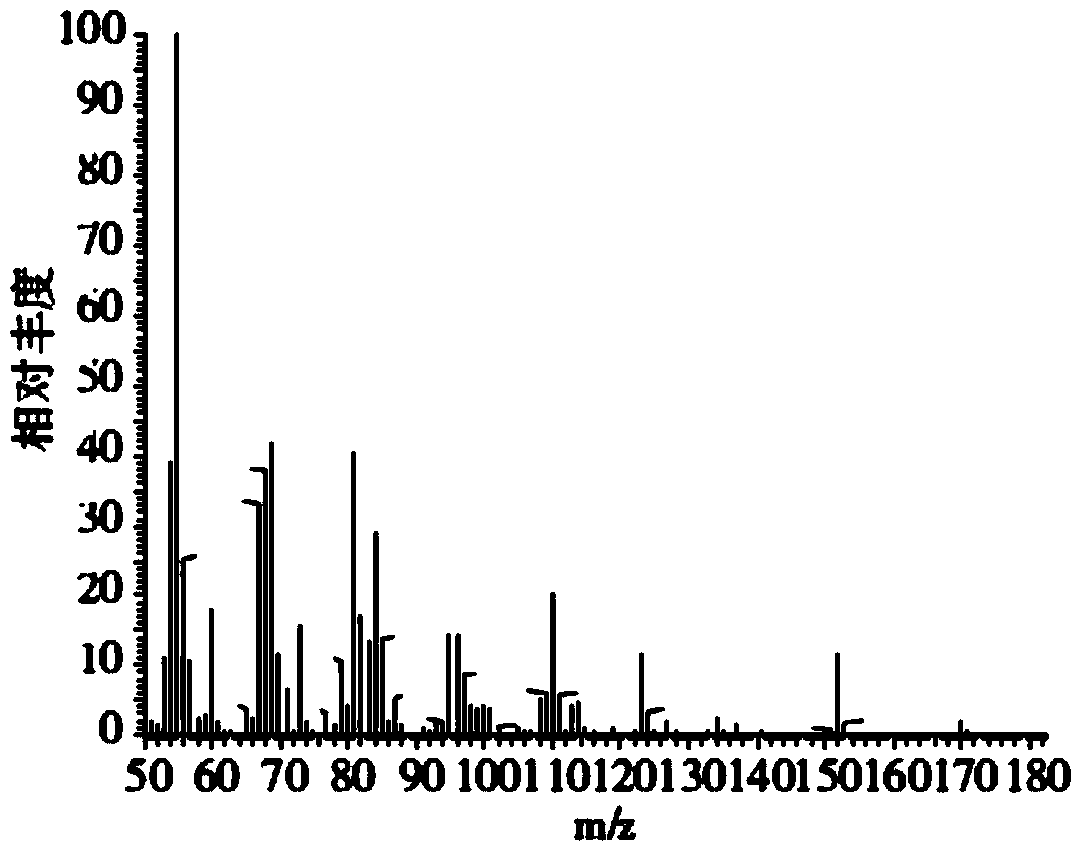
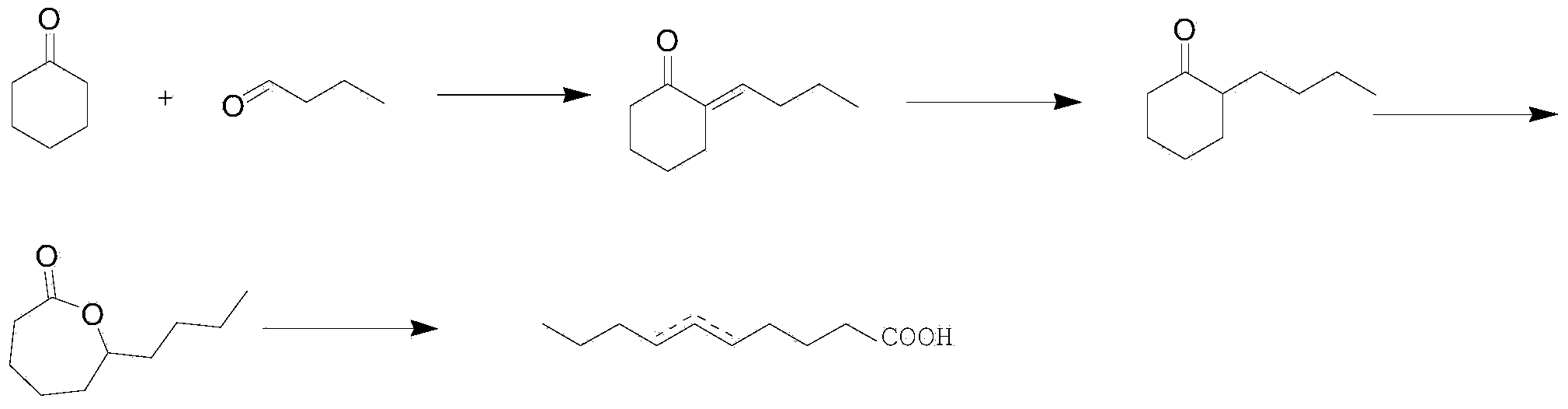
Process for synthesizing milk lactone
A technology of milk lactone and synthesis process, applied in the field of food additives, can solve the problems of high cost, complicated operation, harsh reaction conditions and the like, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic technique of milk lactone, it specifically comprises the following steps:
[0029] 1. Synthesis of 2-butylene cyclohexanone
[0030] In a 500mL three-neck flask equipped with a thermometer, a stirrer, and a dropping funnel, add 180mL of 1% sodium hydroxide solution, 5.40g of polyethylene glycol 400, 176.67g (about 1.8mol) of cyclohexanone, After mixing the above materials evenly, add 86.52 g (about 1.2 mol) of n-butyraldehyde into the three-necked flask dropwise through the dropping funnel, and stir while dropping. After 1 hour, the dropwise addition is completed, and then continue to stir, so that the above reactants are heated at 30 ° C. Under reaction 2h.
[0031] After the reaction, add about 6mL of glacial acetic acid into the three-necked flask, neutralize the water layer to a pH of 6-7, and then separate the oil layer after standing for a while; extract the water layer with 300mL of toluene , and the extracted toluene solution and the oil layer we...
Embodiment 2
[0042]The synthetic technique of milk lactone, it specifically comprises the following steps:
[0043] 1. Synthesis of 2-butylene cyclohexanone
[0044] The difference between this step and Example 1 is that the phase-transfer catalyst used is different, and the phase-transfer catalyst used in this embodiment is cetyltrimethylammonium chloride;
[0045] In a 500mL three-necked flask equipped with a thermometer, a stirrer, and a dropping funnel, add 200mL of 1% sodium hydroxide solution, 5.00g of cetyltrimethylammonium chloride, 196.28g (about 2.0mol ) cyclohexanone, after the above materials are mixed evenly, then dropwise add 86.52g (about 1.2mol) of n-butyraldehyde in the three-necked flask through the dropping funnel, stir while dripping, 1h dropwise, continue to stir subsequently, make the above The reactants were reacted at 30°C for 2h.
[0046] After the reaction, add about 6mL of glacial acetic acid into the three-necked flask, neutralize the water layer to a pH of 6-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com