Method for removing trace iodine in chlor-alkali brine by using ozone
A chlor-alkali brine and ozone technology, applied in the direction of electrolysis components, electrolysis process, etc., can solve the problems of difficult control of reducing agent and oxidant ratio, easy introduction of new impurities, cumbersome operation, etc., to achieve easy control of reaction process, good removal effect, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
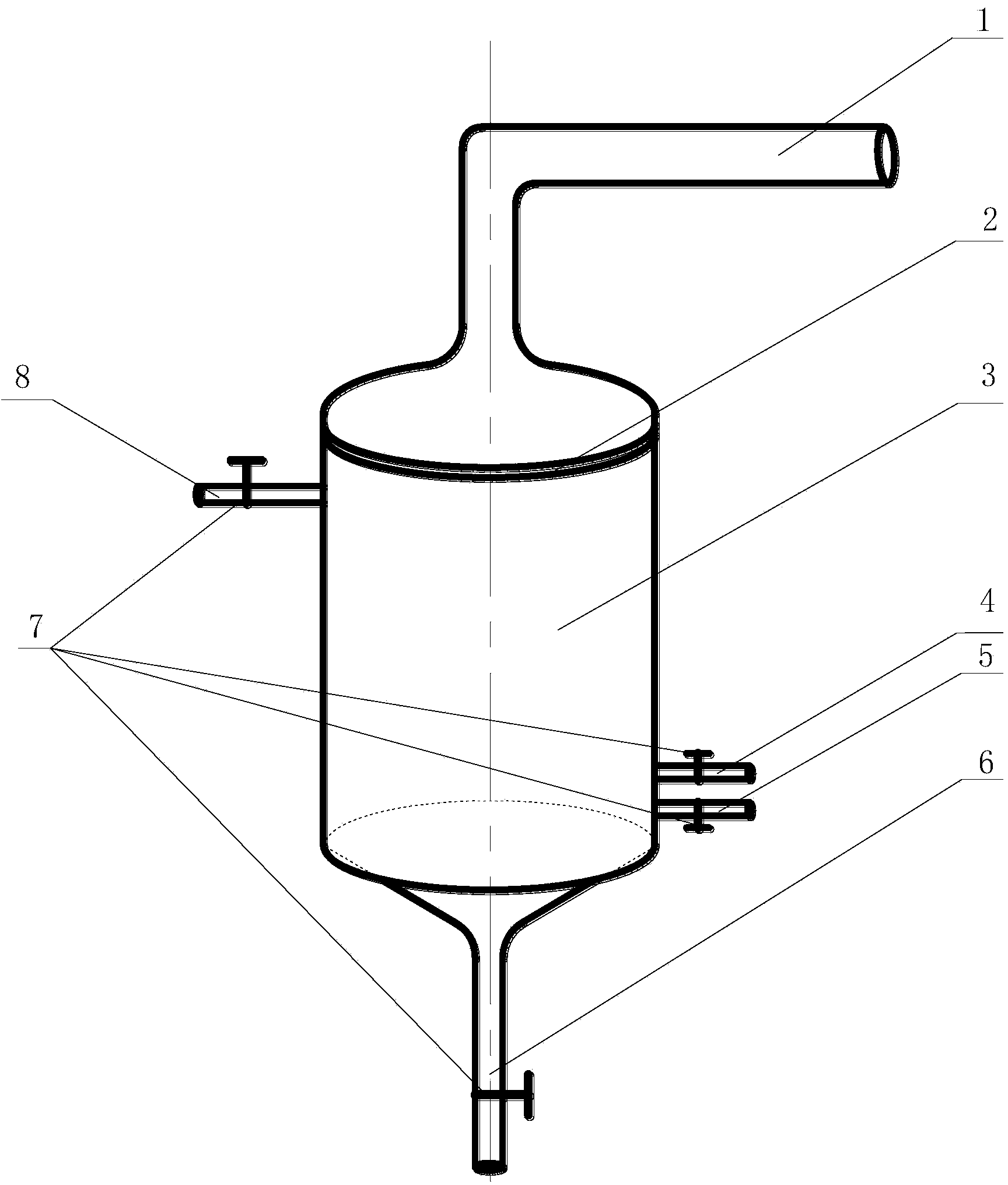
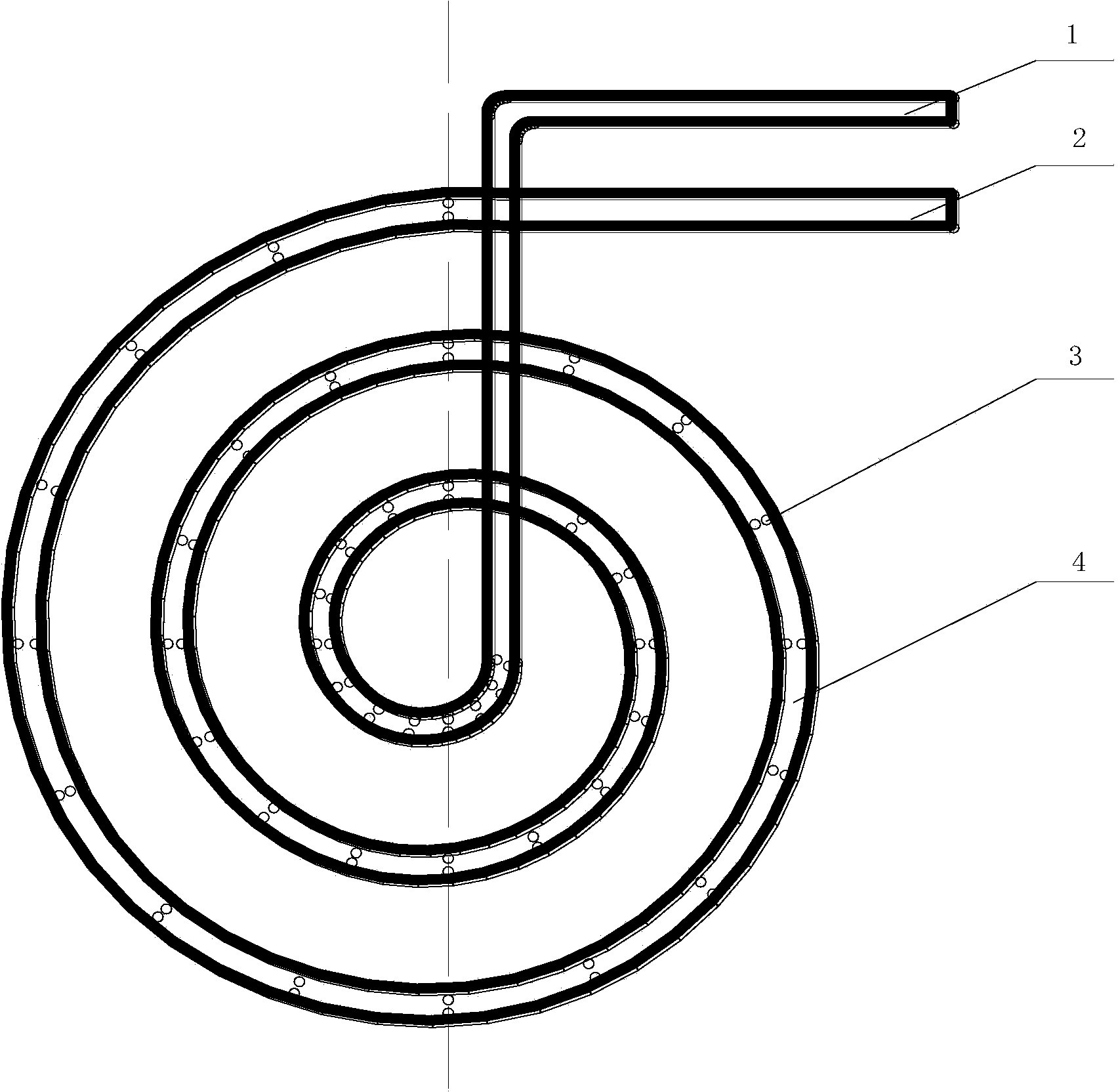
[0035] Factory chlor-alkali brine, the initial iodine content in brine was measured to be 1.96mg / L. Inject brine to a volume of 1m 3 cylindrical reactor. The reactor is equipped with a ventilation coil, and the coil is placed at the bottom of the reactor, and the exhaust holes are evenly distributed on the coil. The brine temperature is controlled at 30°C, and the ozone rate of the coil is 3m 3 / h, after aeration reaction 60min, measure the iodine content in the brine with spectrophotometry to be 0.89mg / L.
Embodiment 2
[0037] Factory chlor-alkali brine, the initial iodine content in brine was measured to be 1.86mg / L. Inject saline into a volume of 1m 3 cylindrical reactor. The reactor is equipped with a ventilation coil, and the coil is placed at the bottom of the reactor, and the exhaust holes are evenly distributed in the coil. The brine temperature is controlled at 30°C, and the ozone rate of the coil is 6m 3 / h, after aeration reaction 80min, measure the iodine content in the brine with spectrophotometry to be 0.73mg / L.
Embodiment 3
[0039] Factory chlor-alkali brine, the measured initial iodine content in brine is 1.66mg / L. Inject saline into a volume of 1m 3 cylindrical reactor. The reactor is equipped with a ventilation coil, and the coil is placed at the bottom of the reactor, and there are small exhaust holes on the coil. Control the brine temperature to 30°C, and the ozone rate to 9m 3 / h, after aeration reaction time 100min, the iodine content measured by spectrophotometry was 0.64mg / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com