Method for increasing oxygen reduction activity of MnOx catalyst in electrode
A catalyst and electrode technology, which is applied in the field of improving the oxygen reduction activity of MnOx catalysts, can solve the problems of ORR activity changes and changes, and achieve the effects of improving oxygen reduction activity, reducing influence, and strong operation controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
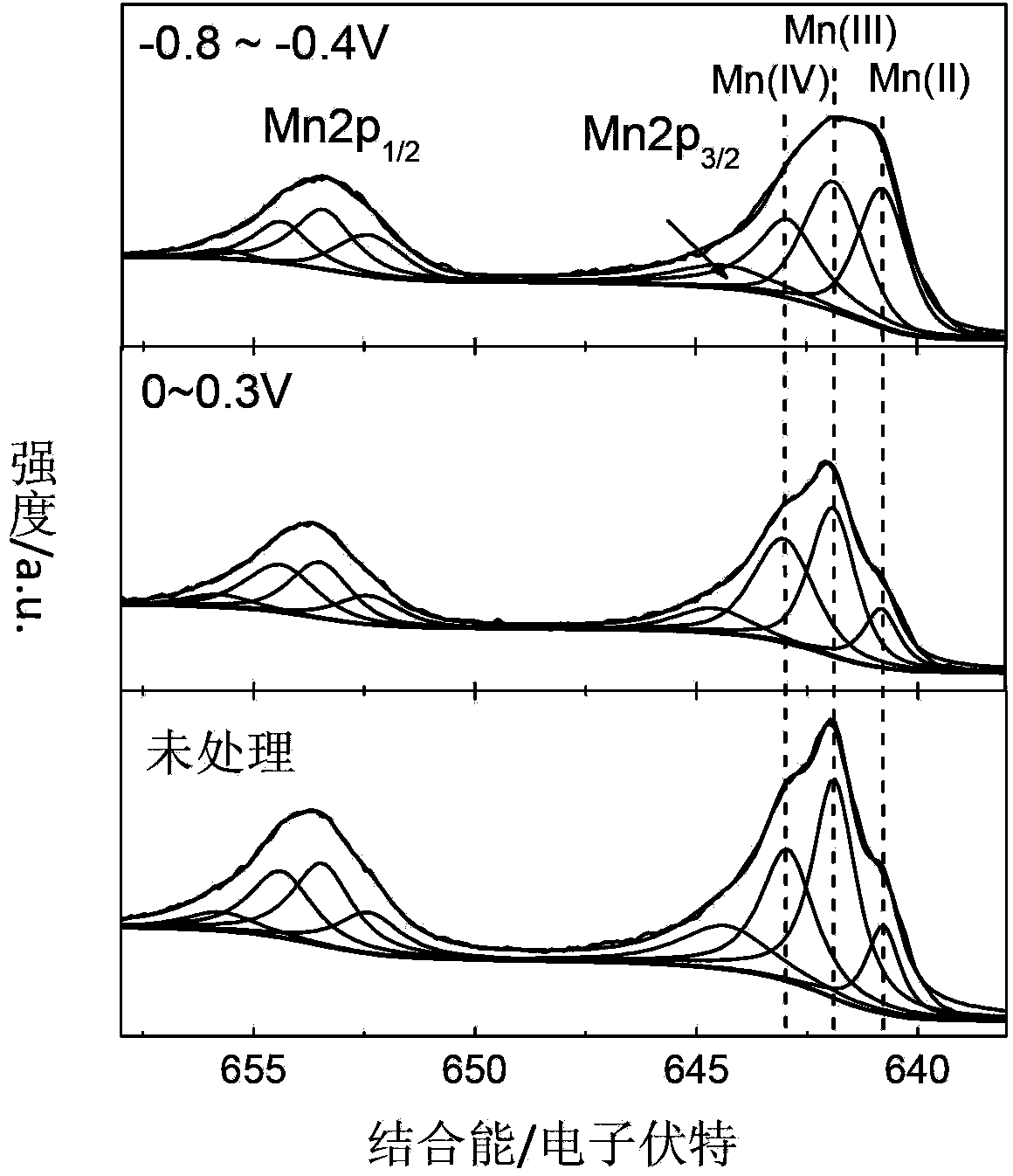
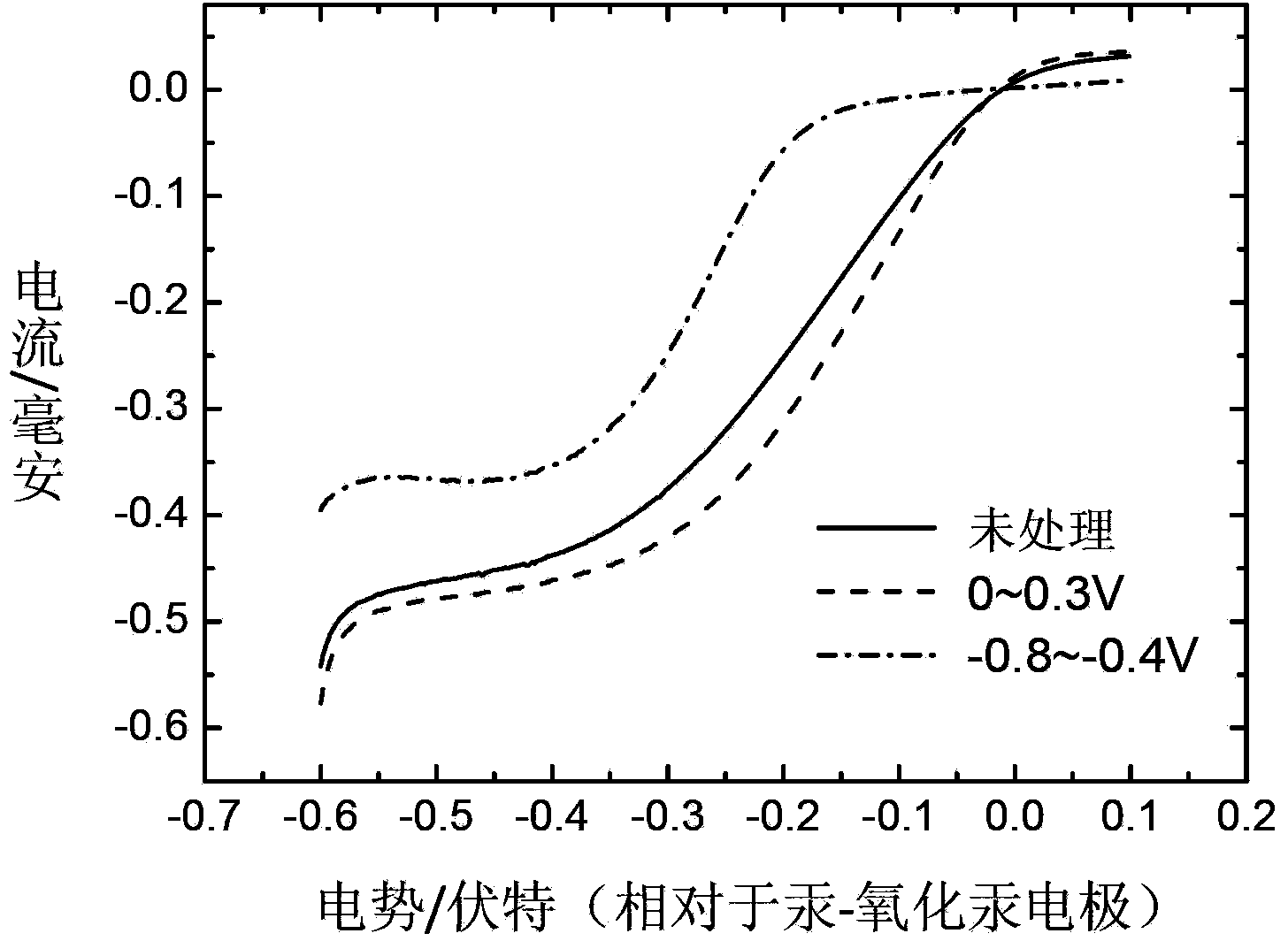
[0027] Weigh 30mg of prepared MnO x As a catalyst, 2 mL of ethanol and 67 mg of Nafion emulsion with a mass fraction of 5% were sequentially added, and the slurry uniformly dispersed by ultrasonic was coated on the surface of a Pt electrode to obtain a working electrode. Take the Pt sheet as the counter electrode and the Hg / HgO electrode as the reference electrode, between 0-0.3V with 10mVs -1 Cyclic voltammetry scan (20 circles) at the scanning speed, the electrode rotation speed is 1600rpn, the half-wave potential of the ORR polarization curve shifts about 30mV, MnO x The catalytic activity for ORR was significantly improved, such as figure 2 shown.
Embodiment 2
[0029] Weigh 30mg of prepared MnO x As a catalyst, 2 mL of ethanol and 67 mg of Nafion emulsion with a mass fraction of 5% were sequentially added, and the slurry uniformly dispersed by ultrasonic was coated on the surface of a Pt electrode to obtain a working electrode. With the Pt sheet as the counter electrode and the Hg / HgO electrode as the reference electrode, the ORR catalytic activity was significantly improved after maintaining the potential at 0.2 V for 5 min.
Embodiment 3
[0031] Weigh 30mg of prepared MnO x As a catalyst, 2 mL of ethanol and 67 mg of Nafion emulsion with a mass fraction of 5% were sequentially added, and the slurry uniformly dispersed by ultrasonic was coated on the surface of a Pt electrode to obtain a working electrode. With Pt sheet as the counter electrode, Hg / HgO electrode as the reference electrode, with 1mVs -1 The scan rate is linearly scanned within the potential window of 0.0-0.4V, and its ORR catalytic activity is significantly improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com