Synthesis method of pyrazolone derivatives by solid acid catalysis
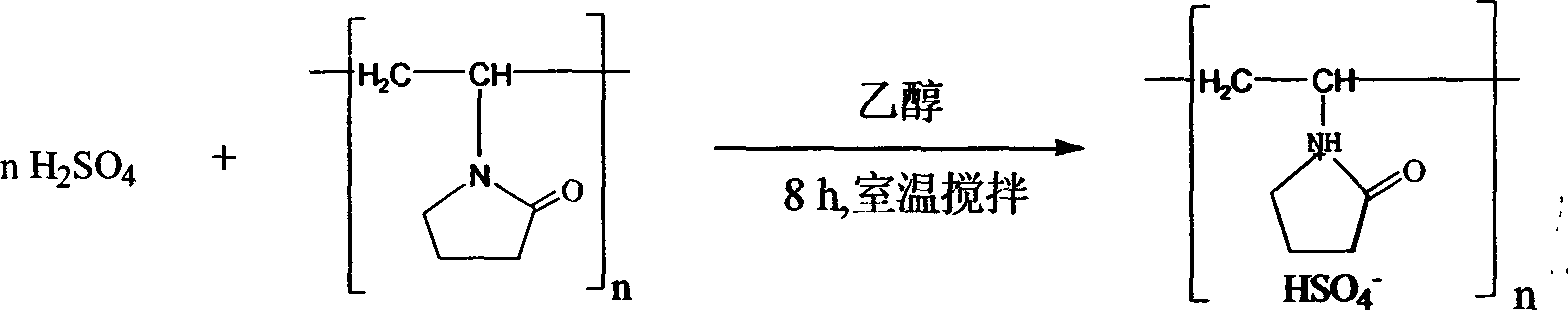
A synthesis method and solid acid technology, which is applied in the field of synthesis of pyrazolone derivatives, can solve the problems of corrosive post-treatment, excessive reagent/catalyst, high temperature and other problems in the reaction process, and achieve recyclable catalyst, non-toxic and cheap catalyst , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
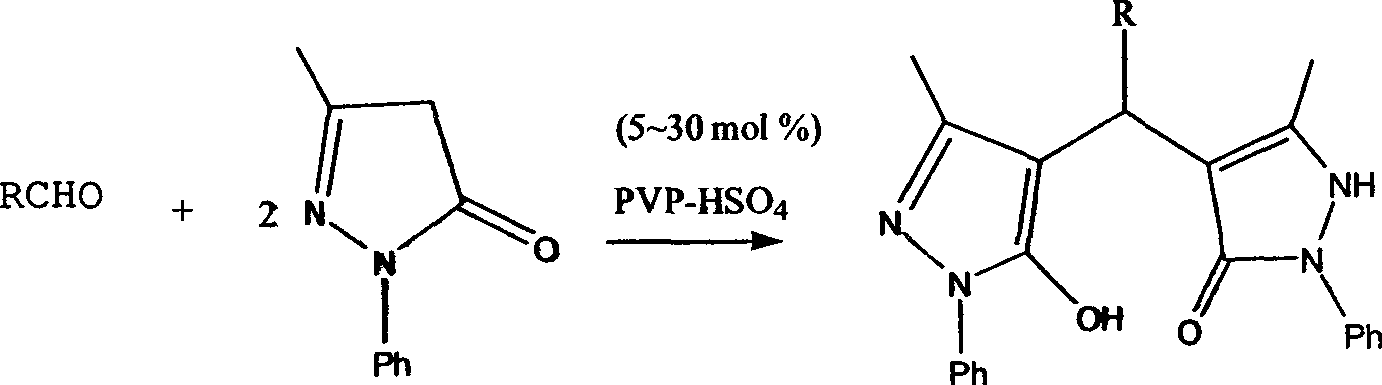
[0018] Example 1 Add 1mmol of benzaldehyde, 2mmol of 1-phenyl-3-methyl-5-pyrazolone, and 0.05mmol of PVP-HSO to a 100ml three-necked flask 4 With 8ml ethanol, react for a certain period of time under reflux conditions, and the reaction is detected by TLC. After the completion of the reaction, add 20ml of water, stir, filter, dry, cross a silica gel column (V 石油醚 :V 乙酸乙酯 =1:2~1:8), to get product 4-[(5-hydroxyl-3-methyl-1-phenyl-1H-4-pyrazolyl)-phenyl-methyl]-5-methyl -2-Phenyl-1,2-dihydro-pyrazolin-3-one, white solid, yield 71%.
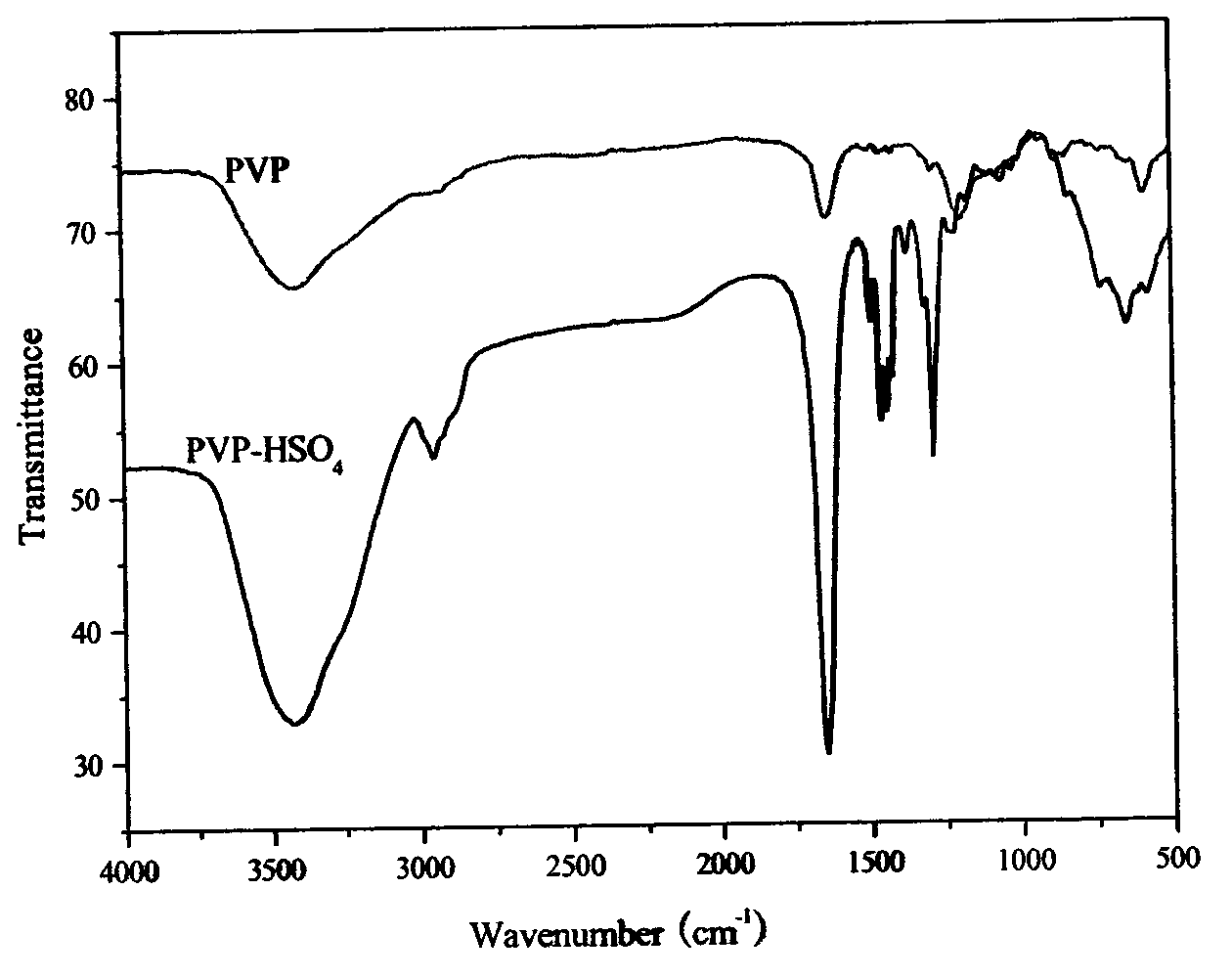
[0019] m.p.=169-171℃; IR (KBr): 3354, 3060, 2930, 2596, 1602, 1575, 1500, 1436, 1371, 1028, 826, 782, 735, 687cm -1 ; 1 H NMR (300MHz, DMSO-d 6 )δ: 1.98(s, 1H, NH), 2.32(s, 6H, 2×CH 3 ), 4.96(s, 1H, CH), 7.23~7.33(m, 7H, ArH), 7.42~7.47(m, 4H, ArH), 7.69~7.72(d, 4H, ArH), 13.79(brs, 1H, OH); MS: M + 436;Anal.calcd for C 27 h 24 N 4 o 2 : C, 74.29; H, 5.54; N, 12.84; Found: C, 74.20; H, 5.45; N, 12.76.
Embodiment 2
[0020] Example 2 Add 1mmol of 2-methoxybenzaldehyde, 2mmol of 1-phenyl-3-methyl-5-pyrazolone, and 0.1mmol of PVP-HSO to a 100ml three-necked flask 4 With 8ml ethanol, react for a certain period of time under reflux conditions, and the reaction is detected by TLC. After the completion of the reaction, add 20ml of water, stir, filter, dry, cross a silica gel column (V 石油醚 :V 乙酸乙酯 =1:2~1:8), to get product 4-[(5-hydroxyl-3-methyl-1-phenyl-1H-4-pyrazolyl)-phenyl-methyl]-5-methyl -2-(2-methoxyphenyl)-1,2-dihydro-pyrazolin-3-one, yellow solid, yield 87%.
[0021] m.p.=210-213℃; IR (KBr): 3448, 3052, 2926, 1592, 1581, 1508, 1410, 1251, 1036, 849, 752, 681cm -1 ; 1 H NMR (300MHz, DMSO-d 6 )δ: 1.98(s, 1H, NH), 2.27(s, 6H, 2×CH 3 ), 3.82 (s, 3H, OCH 3 ), 5.19(s, 1H, CH), 6.87~6.96(m, 2H, ArH), 7.17~7.27(m, 3H, ArH), 7.41~7.46(m, 4H, ArH), 7.58~7.61(d, 1H, ArH), 7.68~7.71 (d, 4H, ArH), 13.95 (brs, 1H, OH); MS: M + 466; Anal.calcd for C 28 h 26 N 4 o 3 : C, 72.09; H, 5.62; N,...
Embodiment 3
[0022] Example 3 Add 1mmol of 4-hydroxybenzaldehyde, 2mmol of 1-phenyl-3-methyl-5-pyrazolone, and 0.2mmol of PVP-HSO to a 100ml three-necked flask 4 With 8ml ethanol, react for a certain period of time under reflux conditions, and the reaction is detected by TLC. After the completion of the reaction, add 20ml of water, stir, filter, dry, cross a silica gel column (V 石油醚 :V 乙酸乙酯 =1:2~1:8), to get product 4-[(5-hydroxyl-3-methyl-1-phenyl-1H-4-pyrazolyl)-phenyl-methyl]-5-methyl -2-(4-Hydroxyphenyl)-1,2-dihydro-pyrazolin-3-one, yellow solid, yield 89%.
[0023] m.p.=157-159℃; IR(KBr): 3425, 3061, 2926, 1602, 1499, 1408, 1292, 810, 752, 674cm -1 ; 1 H NMR (300MHz, DMSO-d 6 )δ: 1.91(s, 1H, NH), 2.29(s, 6H, 2×CH 3 ), 4.85(s, 1H, CH), 6.64~6.67(d, 2H, ArH), 7.02~7.05(d, 2H, ArH), 7.24~7.28(m, 2H, ArH), 7.41~7.47(m, 4H, ArH), 7.69~7.71 (m, 4H, ArH), 9.36 (s, 1H, OH), 13.87 (brs, 1H, OH); MS: M + 452;Anal.calcd for C 27 h 24 N 4 o 3: C, 71.67; H, 5.35; N, 12.38; Found: C, 71...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com