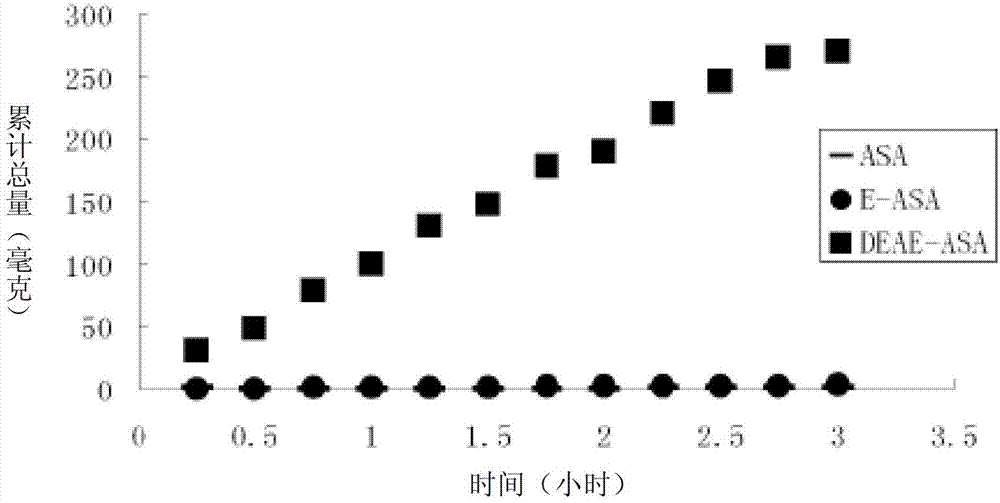
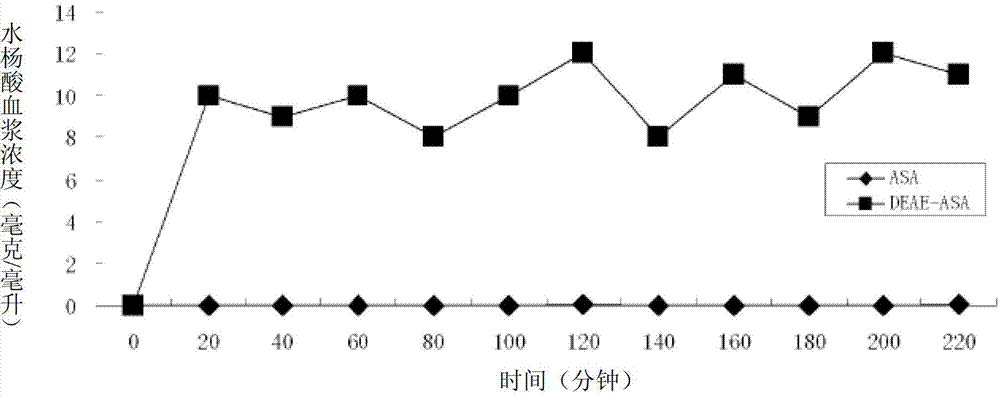
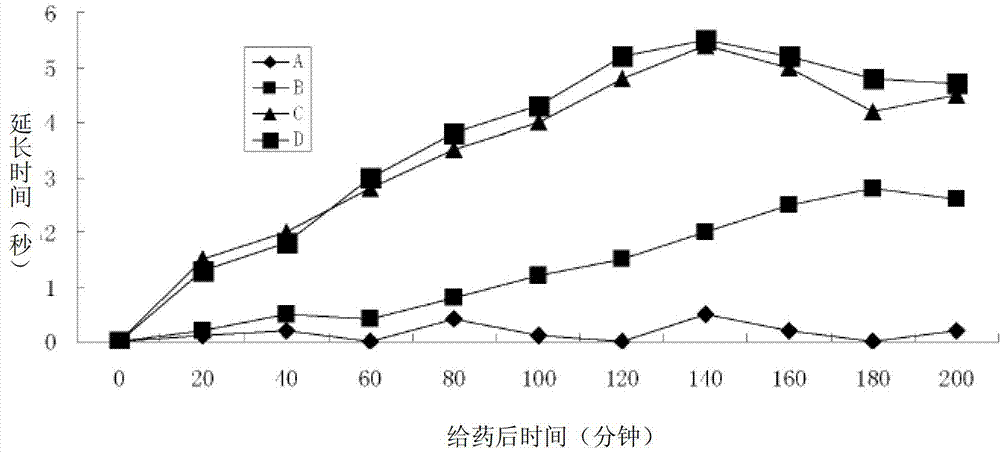
Positively-charged water-soluble prodrugs of aspirin
A kind of technology of aspirin, drug, applied in the field of positively charged water-soluble aspirin prodrug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0060] Preparation of Acetylsalicyloyldimethylaminoethyl Ethyl Acetate
[0061] 19.9 g (0.1 mol) of o-acetoxybenzoyl chloride were dissolved in 100 ml of chloroform. The mixture was cooled to 0°C. 15ml of triethylamine and 8.9g of dimethylaminoethanol were added to the reaction mixture. Stir at room temperature for 3 hours. Stir and add 6 g of acetic acid to the reaction mixture. The solid by-product was filtered off, washed with chloroform (3x30ml), and the organic solvent was evaporated to dryness. After drying, 29 g of the target product (93%) were obtained. Damp product; Solubility: 350mg / ml; Elemental analysis: C 15 h 21 NO 6 ;Molecular weight: 311.33. Theoretical value (%): C: 57.87; H: 6.80; N: 4.50; O: 30.83; found value: C: 57.82; H: 6.85; N: 4.48; 1 H-NMR (400MHz, deuterated chloroform solvent): data: 2.09 (s, 3H) 2.21 (s, 3H), 2.90 (s, 6H); 3.71 (m, 2H), 4.69 (m, 2H), 6.9 ( b, 1H), 7.18 (m, 1H), 7.20 (m, 1H), 7.47 (m, 1H), 7.93 (m, 1H).
[0062] Preparati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com