Application of matrine compounds in preparing anti-hepatic fibrosis and anti-liver cancer medicaments
An anti-hepatic fibrosis and matrine technology, applied in the field of medicine, can solve the problems of poor stability, easy elimination, and affecting the application of anti-hepatic fibrosis drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
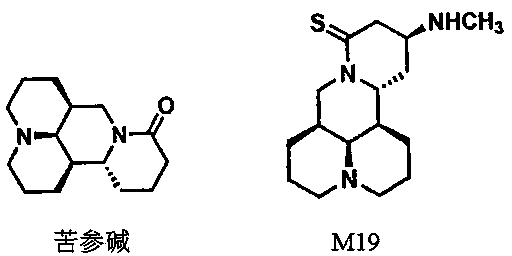
[0034] Embodiment 1: Preparation of 15-thiosophocarpine
[0035] 1.0 g (0.004 mol) of sophocarpine and 2.0 g (0.005 mol, purchased from Alfa Company) of sophocarpine and 2.0 g of Lawson's reagent were placed in a 50 ml reaction bottle, 20 ml of dichloromethane was added, and the reaction was heated under reflux for 12 hours. After the reaction was completed, the solvent was removed by concentration under reduced pressure, and the crude product was passed through a silica gel column with dichloromethane:methanol (25:1) as the eluent to obtain 0.96 g of the product, with a yield of 91.2%.
Embodiment 2
[0036] Embodiment 2: 13-( N -Methyl)-amino-15-thiomatrine (M19) Preparation
[0037] Put 100 mg (0.0004 mol) of 18-thiosophocarpine in a 20 ml reaction bottle, add 2 ml of methylamino alcohol solution and 1 ml of triethylamine, and stir for 12 hours. After the reaction was completed, the solvent was removed by concentration under reduced pressure, and the crude product was passed through a silica gel column with methylene chloride:methanol (20:1) as the eluent to obtain 98 mg of the product with a yield of 83.5%.
Embodiment 3
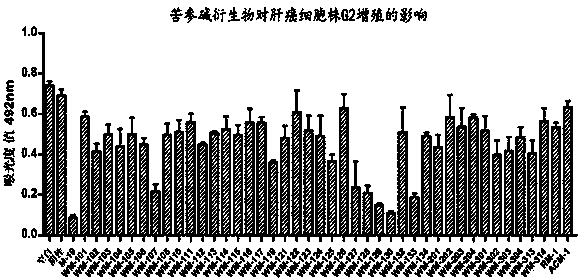
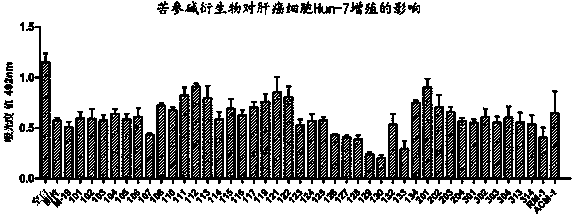
[0038] Embodiment 3: 13-( N -methyl, N Preparation of -chloroacetyl)-amino-18-thiomatrine (WM-1 in the table)
[0039]Add M-19 (2 g, 6.8 mmol) and anhydrous potassium carbonate (1 g, 7.2 mmol) into a round-bottomed flask, vacuumize and inject 40 mL of anhydrous dichloromethane under the protection of Ar balloon. After ice bathing for 10 min, slowly drop 10 mL of anhydrous dichloromethane diluted chloroacetyl chloride (0.62 mL, 7.9 mmol) into the bottle under low temperature and rapid magnetic stirring. h The reaction was completed and monitored by TLC (dichloromethane / methanol, V / V, 10:1). Filter with celite, concentrate the filtrate under reduced pressure, add methanol to solidify and wash. The solid was dissolved in dichloromethane again, and an equal volume of methanol was added, concentrated under reduced pressure until a large amount of solid precipitated, filtered with suction to obtain a relatively pure product, and dried under reduced pressure to obtain 1.1 g, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com