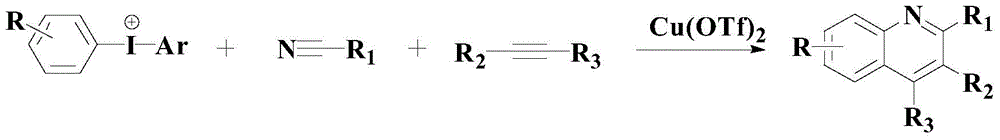A kind of synthetic method of pharmaceutical intermediate polysubstituted quinoline derivatives
A synthesis method and a pharmaceutical technology are applied in the field of synthesis of pharmaceutical intermediate quinoline derivatives, and can solve the problems that the reaction conditions need to be improved, the reaction yield needs to be improved, the scope of application of the reaction raw materials needs to be broadened, and the like, and the effect of improving the harsh reaction is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Add 1mmol of the compound of formula (I) and 2.3mmol of the compound of formula (II) in the closed reaction kettle, then add 4ml of toluene and 4ml of acetonitrile, and then add 0.08mmol of Pd(OAc) under stirring 2 And 6mmol trifluoromethanesulfonic acid, finally add 110mg mass ratio of 1:2.5 1,2-bis(diphenylphosphine)ethane (dppe) and 18-crown-6 additive mixture, heat up to 100°C for reaction 12h, TLC monitors the end point of the reaction, after the reaction is completed, the mixture is added to a saturated sodium bicarbonate solution, then extracted with ethyl acetate, the combined organic phases are washed with water, separated, and concentrated in vacuo, and the residue is purified by silica gel column chromatography to obtain the formula (III) compound, the yield is 98.1%, and the purity is 99.3% (HPLC).
[0038] 1 H NMR (400MHz, CDCl 3)δ=8.17(d,J=8.8Hz,1H),7.64(d,J=8.8Hz,1H),7.53(s,1H),7.41-7.28(m,5H),7.25-7.19(m,3H ), 7.14-7.08 (m, 2H), 7.03-6.96 ...
Embodiment 2
[0040]
[0041] Add 1mmol of the compound of formula (I) and 2mmol of the compound of formula (II) into the closed reaction kettle, then add 4.5ml of toluene and 4.5ml of acetonitrile, and then add 0.05mmol of Pd(OAc) under stirring 2 And 7mmol trifluoromethanesulfonic acid, finally add 100mg mass ratio of 1,2-bis(diphenylphosphine)ethane (dppe) and 18-crown-6 auxiliary agent mixture, heat up to 105°C for reaction 11h, TLC monitors the end point of the reaction, after the reaction is completed, the mixture is added to a saturated sodium bicarbonate solution, then extracted with ethyl acetate, the combined organic phases are washed with water, separated, and concentrated in vacuo, and the residue is purified by silica gel column chromatography to obtain the formula (III) compound, the yield is 99.1%, and the purity is 98.9% (HPLC).
[0042] 1 H NMR (400MHz, CDCl 3 )δ=8.32(m, 1H), 7.74(t, J=7.47Hz, 1H), 7.57(d, J=7.47Hz, 1H), 7.49(t, J=7.47Hz, 1H), 7.37-7.35( m, 2H), 7.26-...
Embodiment 3
[0044]
[0045] Add 1mmol of the compound of formula (I) and 2.5mmol of the compound of formula (II) in the closed reaction kettle, then add 5ml of toluene and 5ml of acetonitrile, and add 0.06mmol of Pd(OAc) successively under stirring 2 And 8mmol trifluoromethanesulfonic acid, finally add 120mg mass ratio of 1:2.5 1,2-bis(diphenylphosphine)ethane (dppe) and 18-crown-6 additive mixture, heat up to 110°C for reaction 12h, TLC monitors the end point of the reaction, after the reaction is completed, the mixture is added to a saturated sodium bicarbonate solution, then extracted with ethyl acetate, the combined organic phases are washed with water, separated, and concentrated in vacuo, and the residue is purified by silica gel column chromatography to obtain the formula (III) compound, the yield is 98.7%, and the purity is 99.0% (HPLC).
[0046] 1 H NMR (400MHz, CDCl 3 )δ=8.25(s,1H),8.19(d,J=8.2Hz,1H),8.03(d,J=8.2Hz,1H),7.91-7.85(m,1H),7.81-7.74(m,2H ), 7.60-7.55 (m, 2H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com