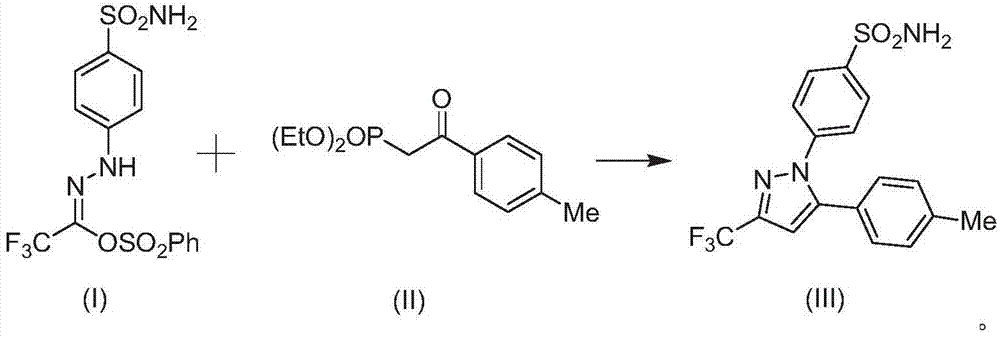
A kind of method of synthesizing celecoxib
A technology for celecoxib and a compound is applied in the field of drug synthesis and preparation, and can solve the problems of being difficult to meet a large number of demands in the field of pharmaceutical synthesis, affecting large-scale production, and unsatisfactory product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of celecoxib
[0026]
[0027] Add 1mmol of the compound of formula (I) and 9g of solvent to the reactor, stir and mix at room temperature for 8min, then add 1.1mmol of the compound of formula (II), continue to stir and mix for 7min, then add 0.11mmol of the catalyst In(OAc) 3 , 0.12mmol ligand, 1.6mmol LiOH and 18mg additives Molecular sieves, stirred at room temperature and reacted for 5h. After the reaction was completed, the compound of formula (III) was obtained by filtration, vacuum concentration, and column chromatography (eluted with n-hexane / ethyl acetate), and the yield was 97.1%.
[0028] Wherein, the solvent is a mixture of ethylene glycol dimethyl ether and N-butylpyridine tetrafluoroborate with a mass ratio of 4:1; the ligand is 9-[2-(dicyclohexylphosphino)phenyl]- 9H-carbazole.
Embodiment 2
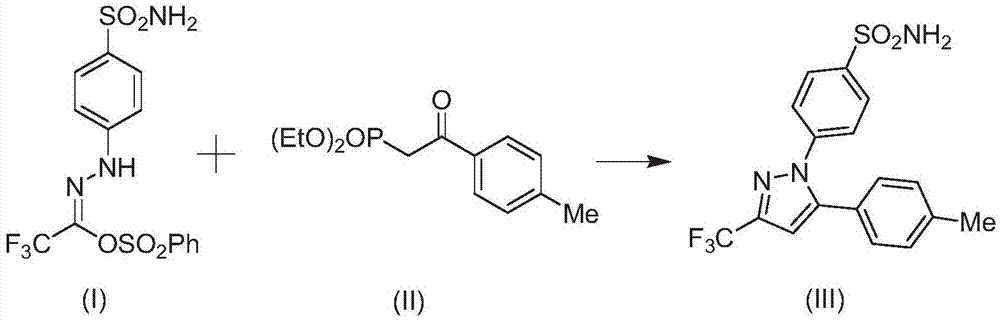
[0029] Embodiment 2: the synthesis of celecoxib
[0030]
[0031] Add 1mmol of the compound of formula (I) and 8g of solvent to the reaction kettle, stir and mix at room temperature for 5min, then add 1.1mmol of the compound of formula (II), continue to stir and mix for 10min, then add 0.1mmol of the catalyst In(OAc) 3 , 0.14mmol ligand, 1.7mmol LiOH and 15mg additives Molecular sieves, stirred at room temperature and reacted for 4h. After the reaction was completed, the compound of formula (III) was obtained by filtration, vacuum concentration, and column chromatography (eluted with n-hexane / ethyl acetate). The yield was 96.7%.
[0032] Wherein, the solvent is a mixture of ethylene glycol dimethyl ether and N-butylpyridine tetrafluoroborate with a mass ratio of 4:1; the ligand is 9-[2-(dicyclohexylphosphino)phenyl]- 9H-carbazole.
Embodiment 3
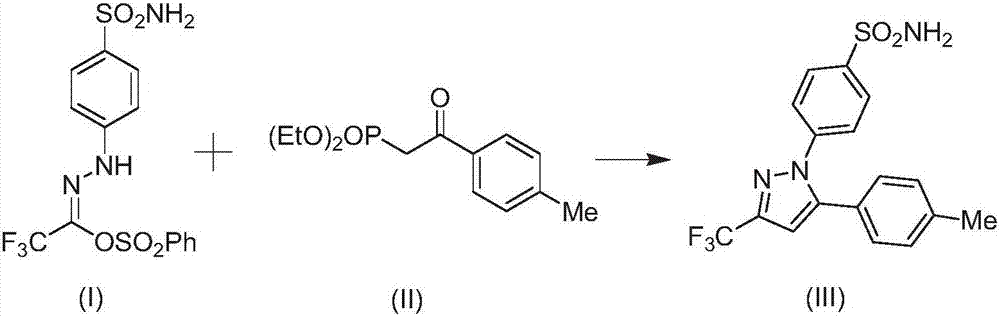
[0033] Embodiment 3: the synthesis of celecoxib
[0034]
[0035] Add 1mmol of the compound of formula (I) and 10g of solvent to the reactor, stir and mix at room temperature for 10min, then add 1.2mmol of the compound of formula (II), continue to stir and mix for 5min, then add 0.12mmol of the catalyst In(OAc) 3 , 0.13mmol ligand, 1.8mmol LiOH and 16mg additives Molecular sieves, stirred at room temperature and reacted for 6h. After the reaction was completed, the compound of formula (III) was obtained by filtration, vacuum concentration, and column chromatography (eluted with n-hexane / ethyl acetate). The yield was 97.3%.
[0036] Wherein, the solvent is a mixture of ethylene glycol dimethyl ether and N-butylpyridine tetrafluoroborate with a mass ratio of 4:1; the ligand is 9-[2-(dicyclohexylphosphino)phenyl]- 9H-carbazole.
[0037] Product Confirmation
[0038] After testing, the parameters of the celecoxib products prepared in all examples are consistent with those r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com