Cefoperazone sodium and tazobactam sodium medicinal composition for injection and preparation method thereof
A technology of cefoperazone sodium and tazobactam sodium, which is applied in the field of medicine, can solve the problems of prolonging the production cycle, taking a long time, increasing costs, etc., and achieve the effects of stable product quality, improved product quality, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
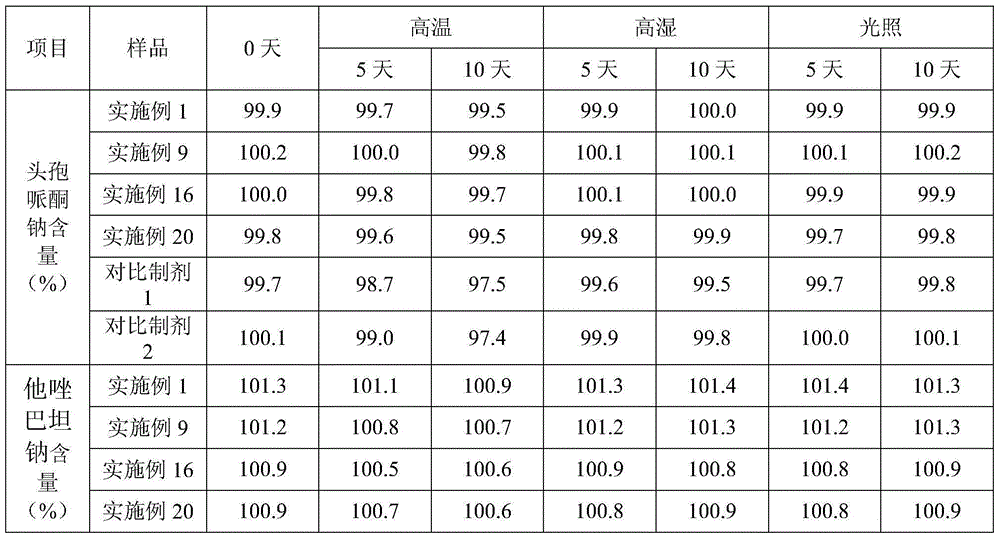
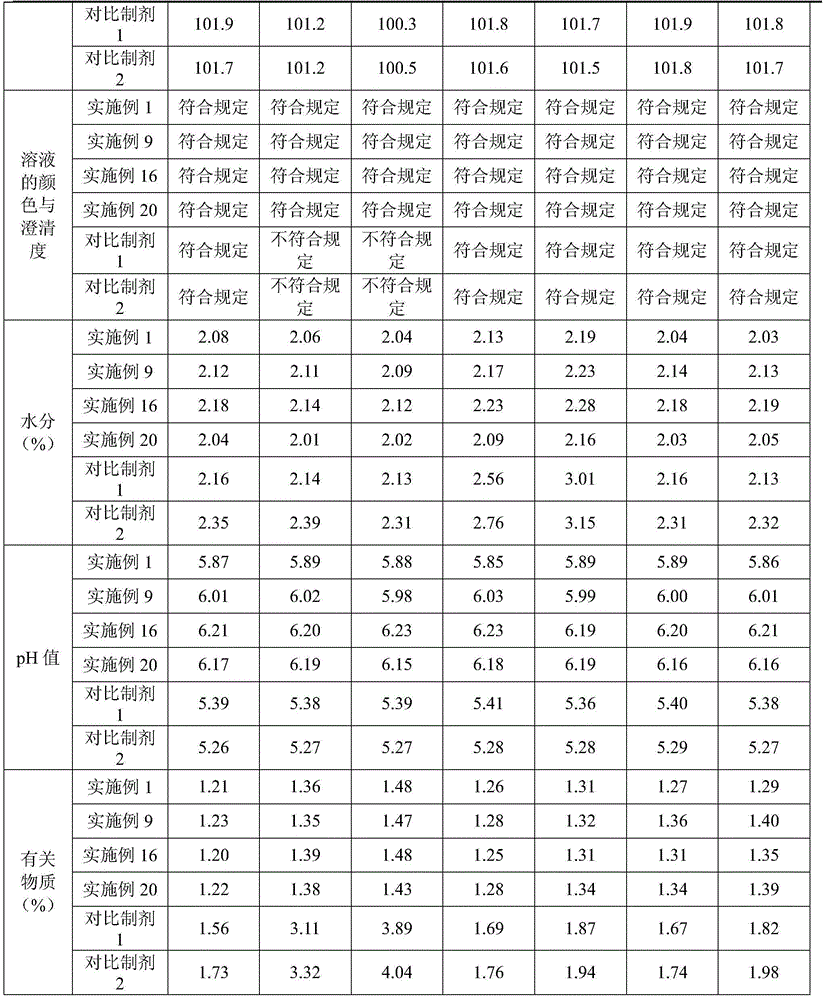
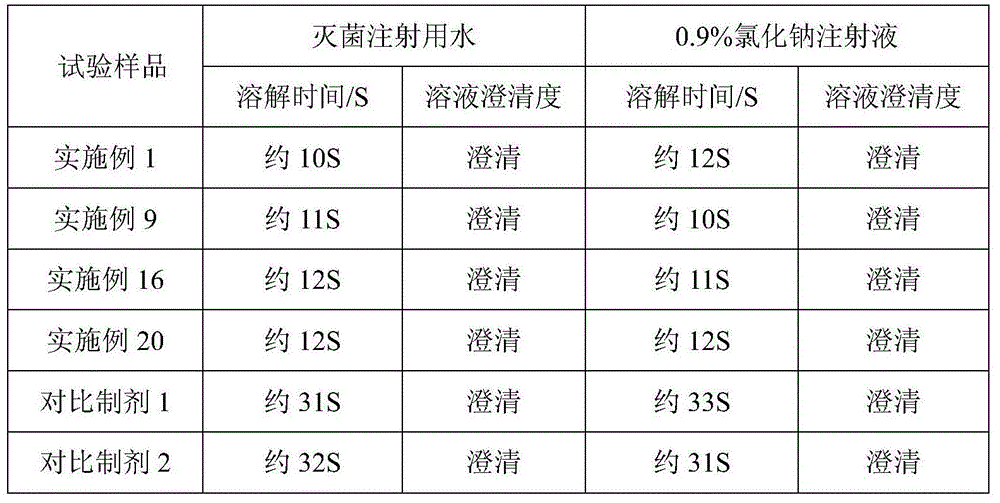
[0036] Example 1 Cefoperazone Sodium and Tazobactam Sodium for Injection (in 1000 pieces, unit: g)
[0037] cefoperazone sodium
[0038] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, cool down to lower the temperature below 20°C, add 2g of polyethylene glycol 15-hydroxystearate, stir until dissolved, then add 200g of cefoperazone sodium and 50g of tazoba Stir the sodium tannin until dissolved; adjust the pH to 6.01 with 1% meglumine solution, add water for injection lower than 20°C to the full amount, fine filter with a 0.22 μm microporous membrane until clear, and conduct semi-finished product inspection; Sealed in a vial, freeze-dried; crimped, sealed, and packaged.
Embodiment 2
[0039] Example 2 Cefoperazone Sodium and Tazobactam Sodium for Injection (in 1000 pieces, unit: g)
[0040] cefoperazone sodium
[0041] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, cool down to lower the temperature below 20°C, add 4g of polyethylene glycol 15-hydroxystearate, stir until dissolved, then add 200g of cefoperazone sodium and 50g of tazoba Stir sodium tannin until dissolved; use 1% meglumine solution to adjust the pH to 6.25, then add water for injection lower than 20°C to the full amount, fine filter with a 0.22 μm microporous membrane until clear, and conduct semi-finished product inspection; Sealed in a vial, freeze-dried; crimped, sealed, and packaged.
Embodiment 3
[0042] Example 3 Cefoperazone Sodium and Tazobactam Sodium for Injection (in 1000 pieces, unit: g)
[0043] cefoperazone sodium
[0044] Preparation process: Inject 500ml of water for injection into the liquid mixing tank, cool down to lower the temperature below 20°C, add 10g of polyethylene glycol 15-hydroxystearate, stir until dissolved, then add 200g of cefoperazone sodium and 50g of tazoba Stir sodium tannin until dissolved; use 1% meglumine solution to adjust the pH to 6.93, then add water for injection lower than 20°C to the full amount, fine filter with a 0.22 μm microporous membrane until clear, and conduct semi-finished product inspection; Sealed in a vial, freeze-dried; crimped, sealed, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com