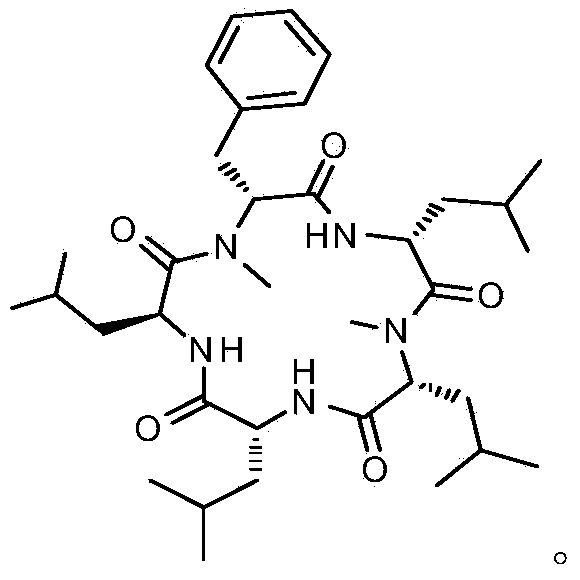
Cyclic pentapeptide as well as synthetic method and application thereof
A synthetic method, a technique of cyclic pentapeptide, applied in the field of organic chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
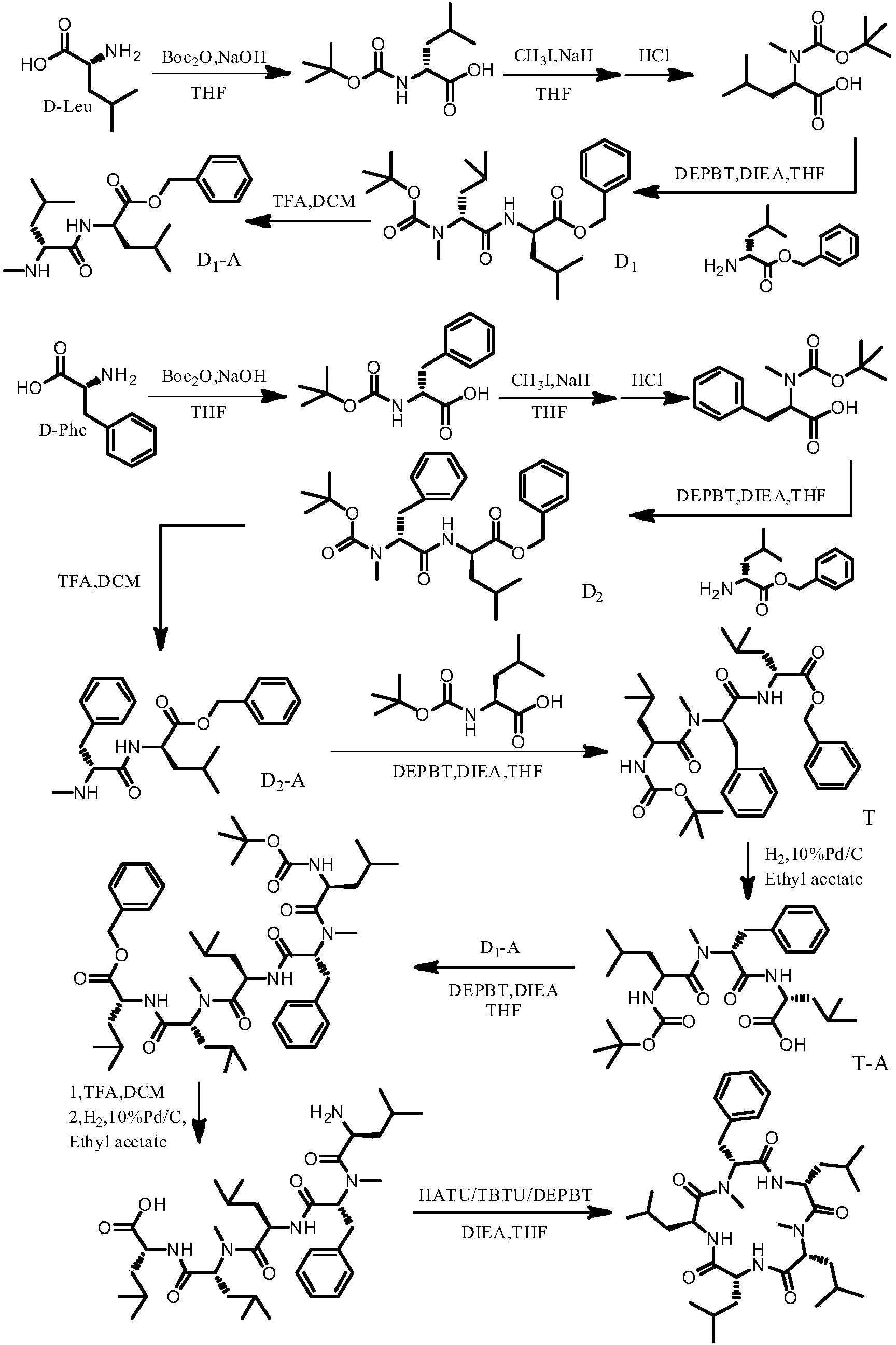
[0048] Example 1 Synthesis of tert-butoxycarbonyl-L-leucine (Boc-L-Leu-OH)
[0049] Under nitrogen protection, tetrahydrofuran (THF, 150 mL), H 2 O (100mL) and 1.0mol / L NaOH solution (150mL), add L-leucine (50mmol, 6.55g) in one batch under cooling in an ice-water bath, stir to dissolve evenly, and then add Boc 2 O (60mol, 13.40g), continue to stir in an ice-water bath for 10 minutes, then slowly rise to room temperature, adjust the pH to 10-10.5, stir overnight, evaporate the solvent under reduced pressure, extract with anhydrous ether, and cool the water phase in an ice-water bath and acidified with 1mol / L hydrochloric acid to pH = 2, then extracted 3 times with ethyl acetate, combined the organic phases and washed with saturated brine, anhydrous MgSO 4 After drying and filtering, the solvent was distilled off to obtain white powder tert-butoxycarbonyl-L-leucine with a yield of 98.7%.
Embodiment 2
[0050] Example 2 Synthesis of tert-butoxycarbonyl-D-leucine (Boc-D-Leu-OH)
[0051] The operation of Example 1 was repeated, except that D-leucine was used instead of L-leucine to obtain white powder tert-butoxycarbonyl-D-leucine with a yield of 99.2%.
Embodiment 3
[0052] Example 3 Synthesis of tert-butoxycarbonyl-D-phenylalanine (Boc-D-Phe-OH)
[0053] The operation of Example 1 was repeated except that D-phenylalanine was used instead of L-leucine to obtain transparent oily tert-butoxycarbonyl-D-phenylalanine with a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com