Pharmaceutical uses of indole piperazine derivatives
A use, piperidine technology, applied in the field of nervous system disease drugs, can solve the problem of not reflecting the real active conformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15-HT1
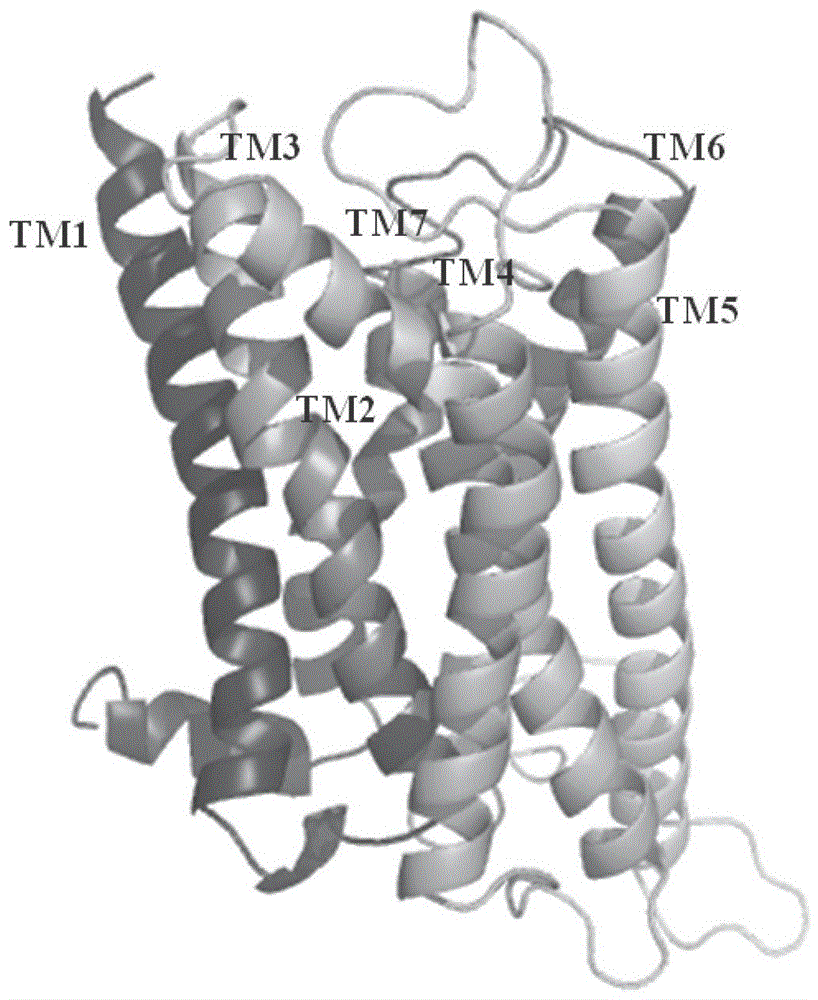
[0026] Example 15-HT 1A Receptor 3D model construction
[0027] The protein sequence is from the Swiss-Prot database (http: / / www.expasy.ch / sprot / ), human epinephrine β 2 receptors and 5-HT 1A The sequence numbers of the receptors are P07550 and P08908, respectively. Using the ClustalW algorithm in the FASTA program, the 5-HT 1A receptors and adrenergic beta 2 The sequence of the receptor was compared by multiple sequences, and then the matching sequence was fine-tuned according to the positions of the conserved residues in the GPCR superfamily, the results are shown in Table 1; 5-HT 1A receptors and adrenergic beta 2 The homology of receptor transmembrane helix is 41.9%.
[0028] Table 15-HT 1A receptors and adrenergic beta 2 Homology and similarity of seven transmembrane domains of receptors
[0029]
[0030] adrenaline beta 2The receptor (RasmussenSGF, ChoiHJ, RosenbaumDM, KobilkaTS, ThianFS, EdwardsPC, etal.Nature2007;450(7168):383-U4.PDB number is 2RH1) was ...
Embodiment 25-HT1
[0031] Example 25-HT 1A Molecular Dynamics Simulation of Receptor Added Membrane
[0032] Molecular dynamics simulations were performed using GROMACS3.3.1 and GROMOS96 force fields. 5-HT after initial energy optimization 1A The receptor model is inserted vertically into the POPC (palmitoyl-oleoyl-phosphatidylcholine) phospholipid bilayer. The protein and POPC structural models are dissolved in the single point charge (SPC) water molecule model; the Berendsen constant temperature method is used to maintain the system temperature at 300K; the system pressure is maintained at one atmospheric pressure; the LINCS algorithm is used to limit the bond length of all chemical bonds containing hydrogen atoms; The electrostatic interaction is calculated by the particle-meshEwald (PME) method, with 9 is the cutoff value (cutoff); the cutoff value of the Lennard-Jones interaction is selected as 14 For each simulation system, a cubic box is used as the simulation unit, the protein is p...
Embodiment 3
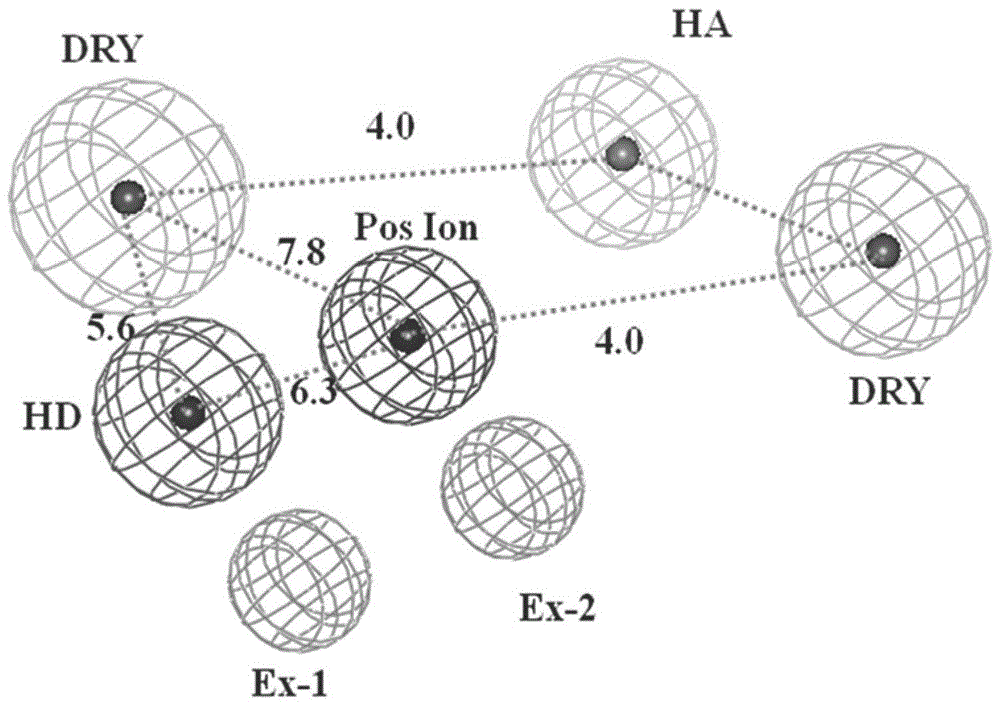
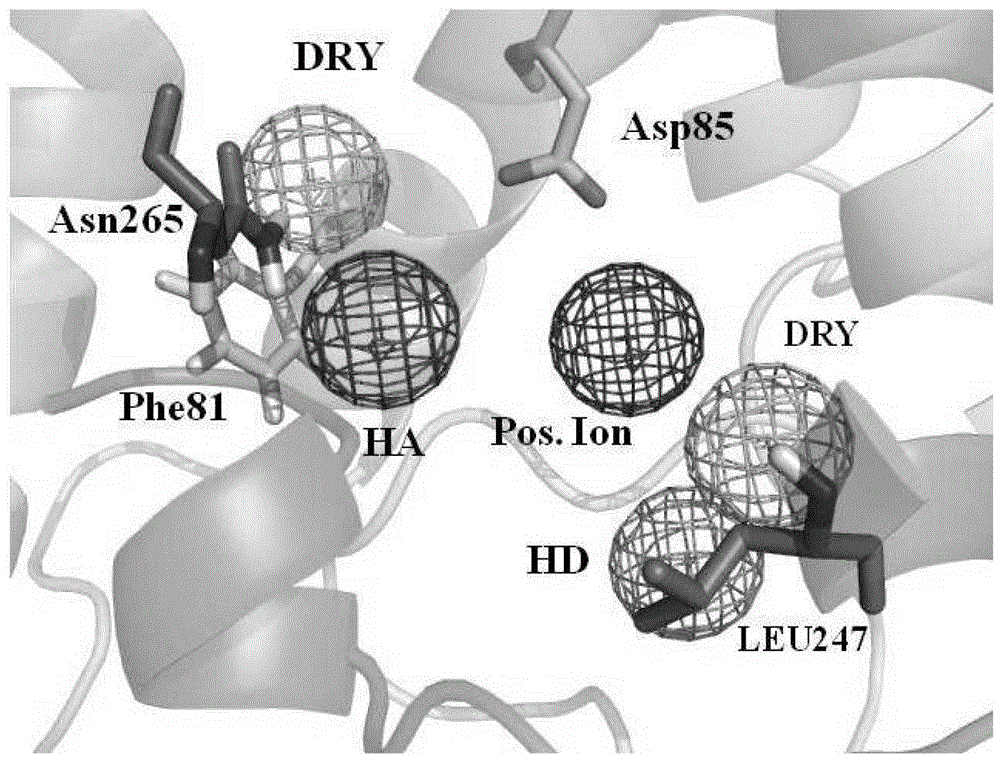
[0034] Embodiment 3 constructs 5-HT 1A Active site detection and dynamic pharmacophore modeling of receptors
[0035] The representative conformations of the first 10 clusters in the kinetic simulation results were taken as the receptor model, and the conserved Asp 3.32 Around 10 The in-scope regions are defined as boxes for site probing by the GRID program. Since the GPCR receptor pocket is negatively charged, four typical probes were selected: N+ (positive charge probe), O (hydrogen bond acceptor probe), N1 (hydrogen bond donor probe) and DRY (hydrophobic group probe). needles) to probe the corresponding electronegativity, hydrogen bond donor, hydrogen bond acceptor, and hydrophobic chemical environment in the protein, respectively. According to the probe point obtained by superimposing 10 conformations of Cα, select the appropriate cluster according to the binding energy of the GRID probe point and the nature of the interaction with the receptor amino acid residue, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com