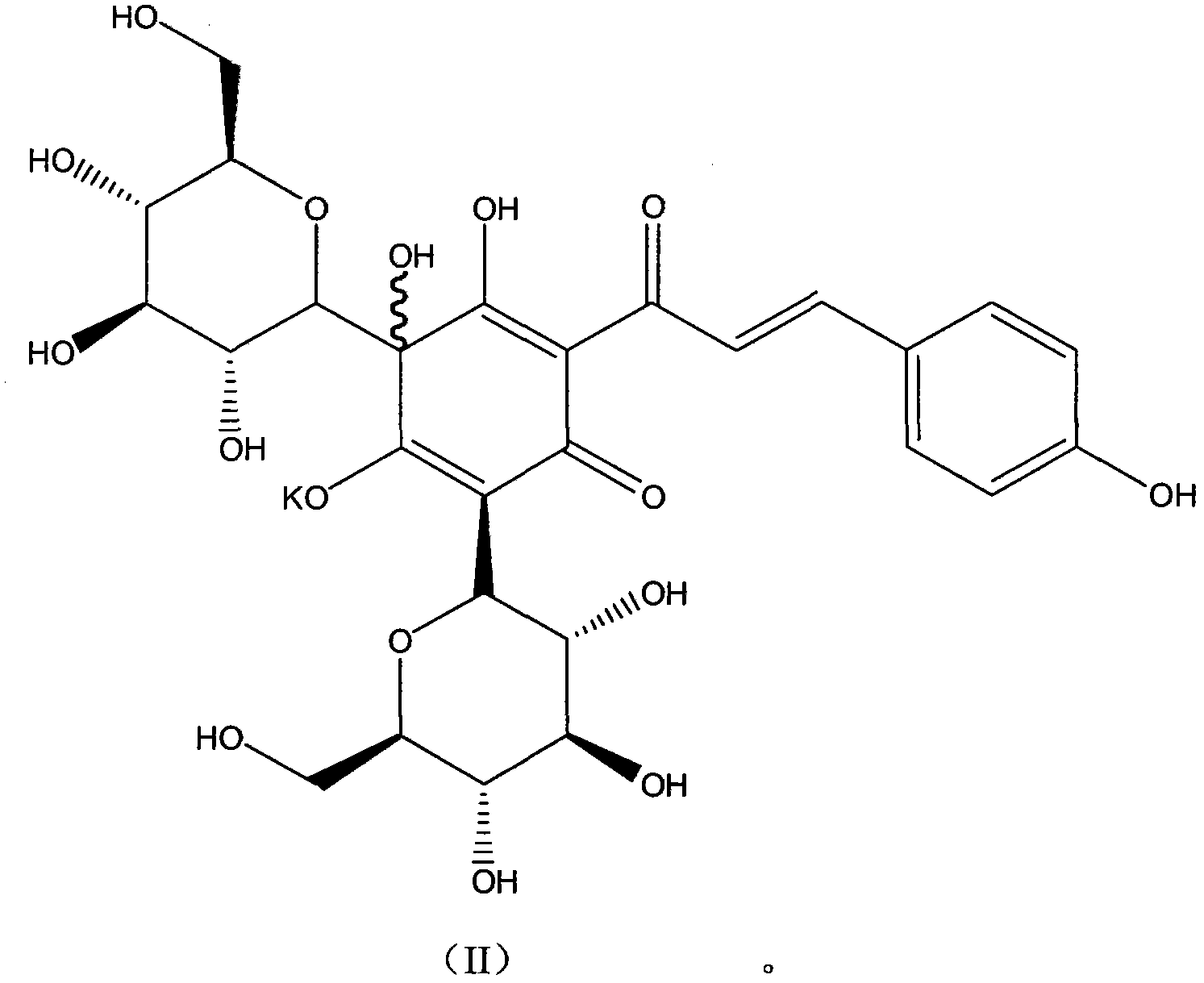
New hydroxysafflor yellow pharmaceutical salt
A technology of hydroxysafflower and yellow pigment, which is applied in freeze-dried powder injection, medicinal salt of hydroxysafflower yellow pigment, medicinal salt of hydroxysafflower yellow A and its preparation field, which can solve the problem of low product purity and influence on product , structural properties can not be qualitative and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
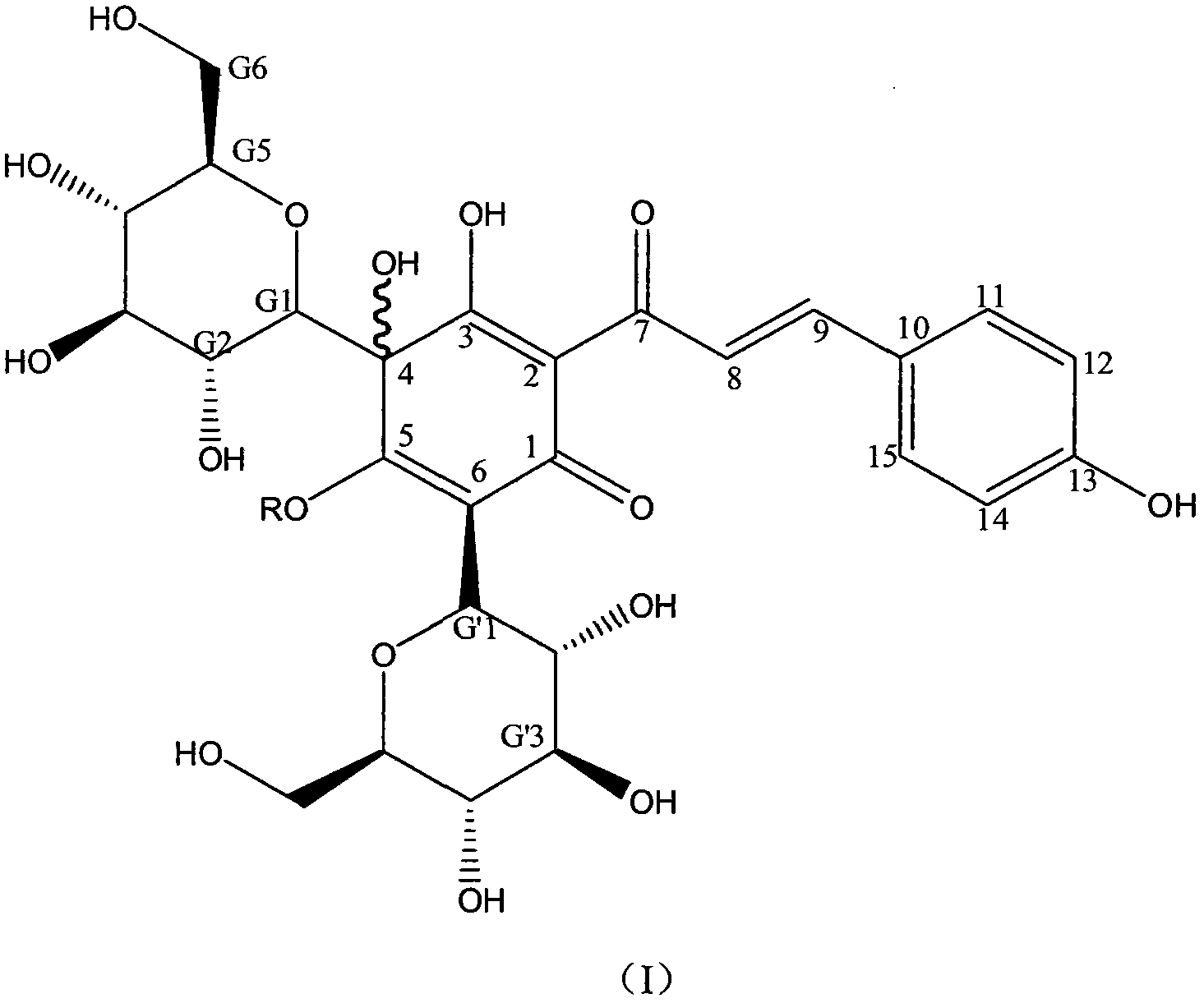
[0111] Embodiment 1: Hydroxy safflower yellow A potassium (in general formula I, R is potassium)
[0112] Weigh safflower, add 12.5 times the weight of the medicinal material in deionized water, extract at 100°C for 20-25 minutes, filter, add 10 times the weight of the medicinal material to the filter residue and repeat the extraction once again according to the above conditions, and filter. Combine the secondary extracts, cool to room temperature, centrifuge with a centrifuge, and take the centrifugate for later use. Slowly add the above centrifugate to the well-balanced 001*7 strongly acidic H-type cation exchange resin, the ratio of column diameter to height is 1:10, the column volume is 500ml, and the flow rate is 3ml / min. Adjust the pH to 4-7, then slowly add to the macroporous adsorption resin separation column, the ratio of column diameter to height is 1:12, and the sample loading flow rate is 10ml per minute. After loading the sample, it was eluted with deionized wate...
Embodiment 2
[0133] Embodiment 2: Hydroxy safflower yellow A potassium (in formula I, R is potassium)
[0134]Weigh safflower, add 12.5 times the weight of the medicinal material in deionized water, extract at 100°C for 20-25 minutes, filter, add 10 times the weight of the medicinal material to the filter residue and repeat the extraction once again according to the above conditions, and filter. Combine the secondary extracts, cool to room temperature, centrifuge with a centrifuge, and take the centrifugate for later use. Slowly add the above centrifugate to the HB-8 macroporous strongly acidic H-type cation exchange resin that has been treated and balanced. The column diameter-to-height ratio is 1:10, the column volume is 500ml, and the flow rate is 3ml / min. Use KOH to adjust the pH to 4-7, then slowly add to the macroporous adsorption resin separation column, the column diameter-to-height ratio is 1:12, and the sample loading flow rate is 10ml per minute. After loading the sample, it wa...
Embodiment 3
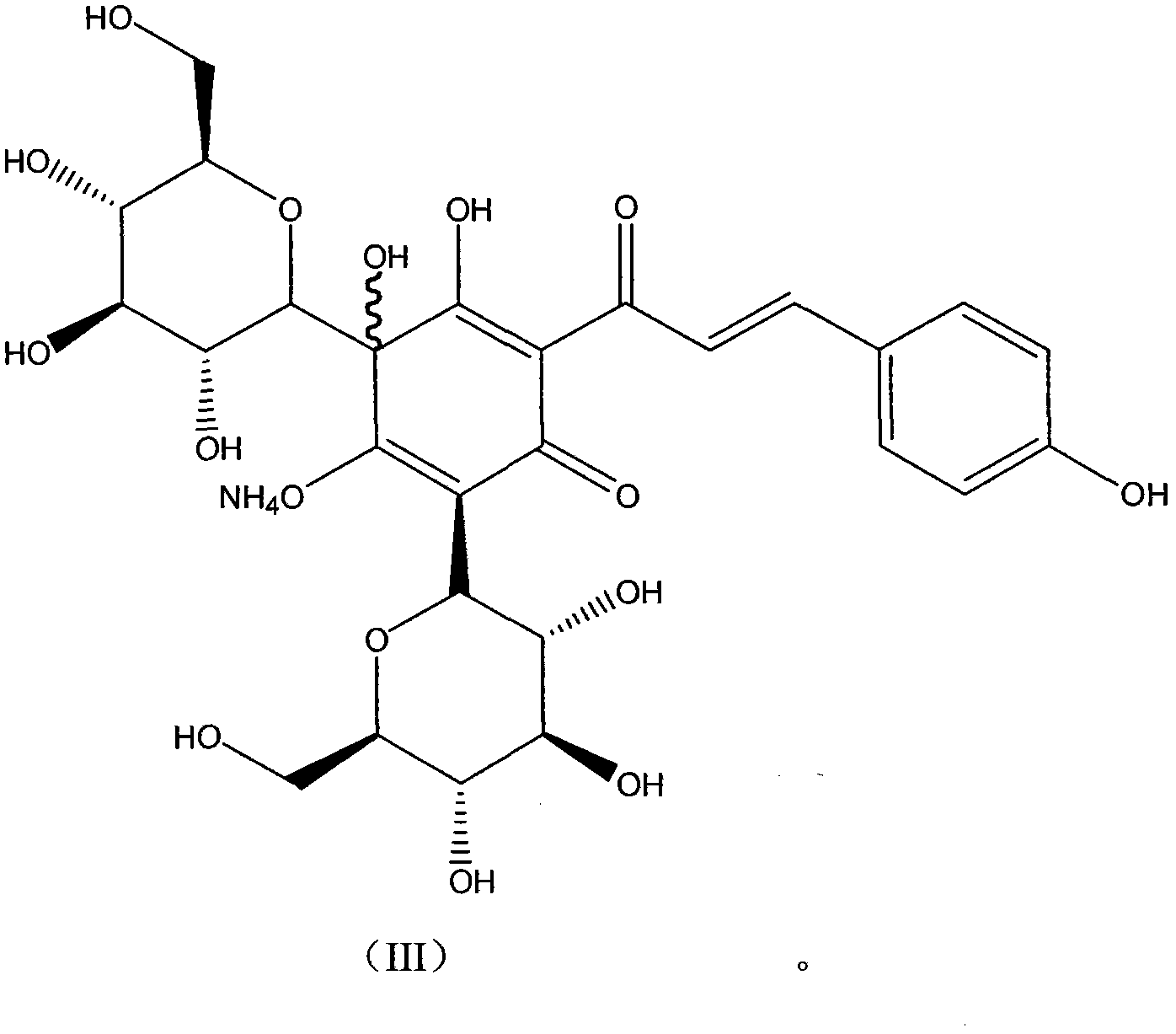
[0135] Embodiment 3: the hydroxyl safflower yellow A ammonium of formula III (in formula I, R is NH 4 )
[0136] Weigh safflower, add 12.5 times the weight of the medicinal material in deionized water, extract at 100°C for 20-25 minutes, filter, add 10 times the weight of the medicinal material to the filter residue and repeat the extraction once again according to the above conditions, and filter. Combine the secondary extracts, cool to room temperature, centrifuge with a centrifuge, and take the centrifugate for later use. Slowly add the above centrifugate to the 001*7 strong acidic H-type cation exchange resin that has been treated and balanced. The column diameter-height ratio is 1:10, the column volume is 500ml, and the flow rate is 3ml / min. Adjust the pH to 4-7, then slowly add to the macroporous adsorption resin separation column, the ratio of column diameter to height is 1:12, and the sample loading flow rate is 10ml per minute. After loading the sample, it was elute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com