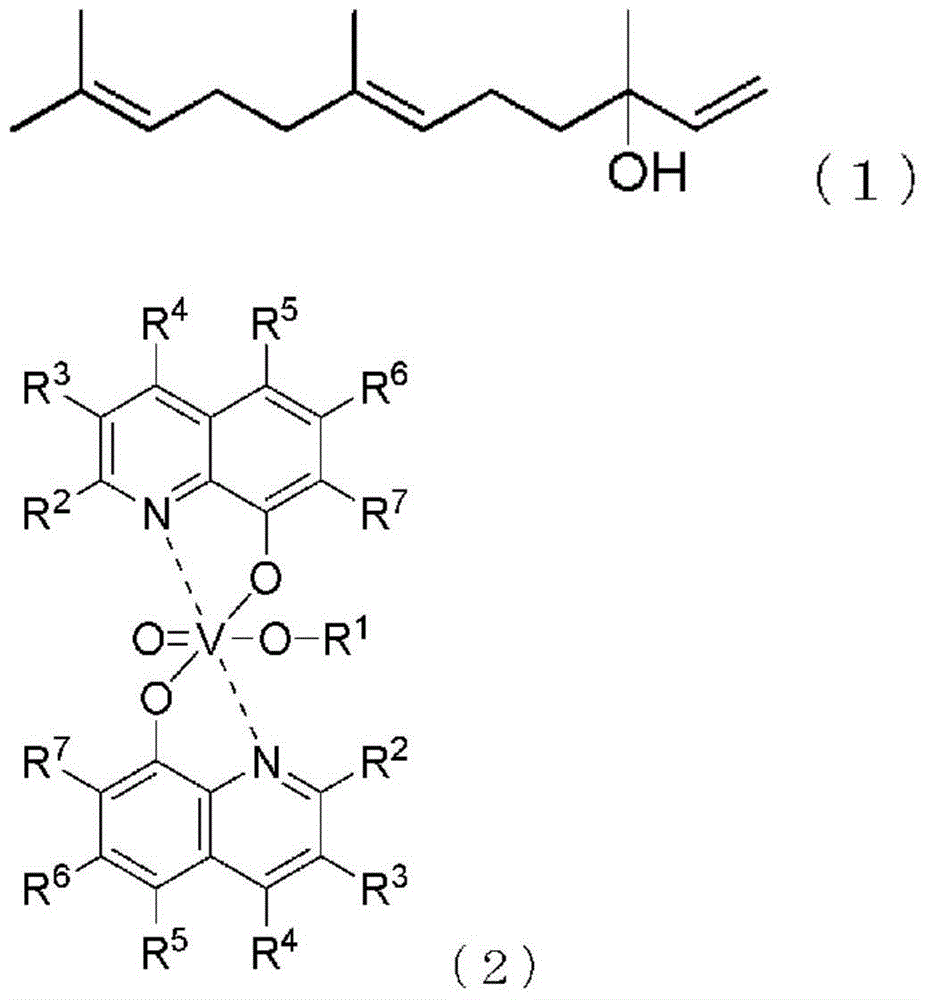
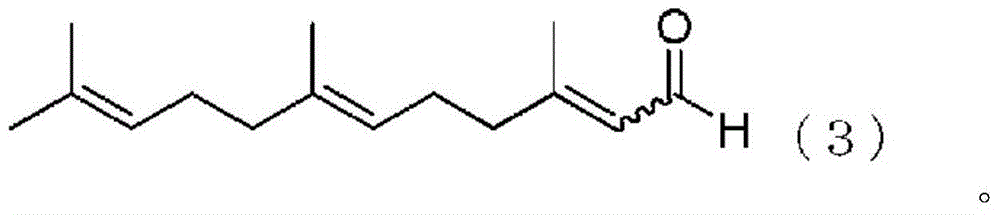
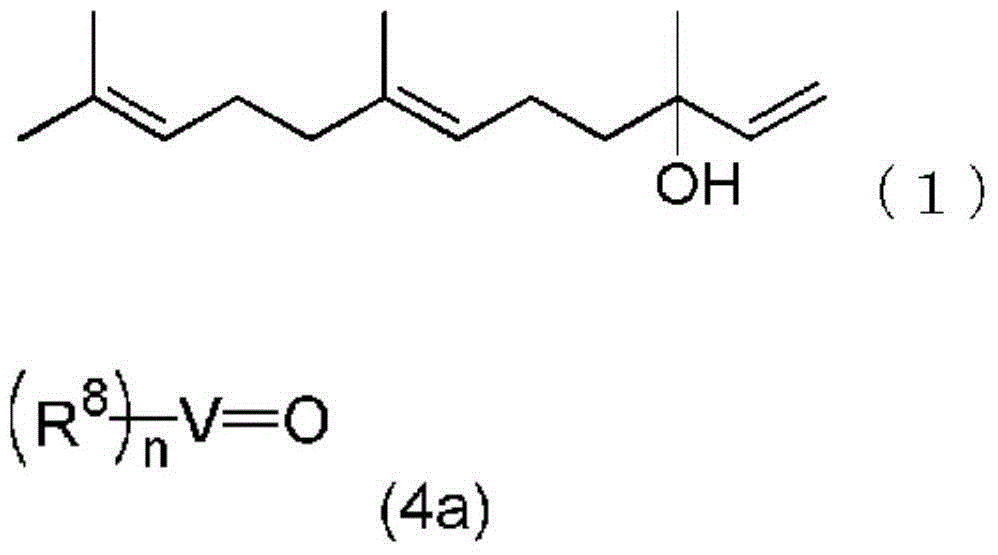
Method for producing farnesal using vanadium complex
A manufacturing method and technology of farnesal, which is applied in the field of new vanadium complexes and its manufacture, can solve the problems of farnesal being expensive, poor in efficiency, impossible to implement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0111] Examples are given below to clarify the embodiment of the present invention, but the present invention is not limited to the examples shown here.
[0112] The reaction solution obtained in the examples was analyzed by gas chromatography, and the purity of (2E, 6E)-farnesal and (2Z, 6E)-farnesal was calculated by area percentage. Measurement conditions are as follows.
[0113]Device: GC-14A (Shimadzu Corporation)
[0114] Column: HP-ULTRA1 (Agilent Technologies)
[0115] 25m×I.D.0.32mm, 0.52μmdf
[0116] Column temperature: 100°C→[10°C / min]→250°C
[0117] Injection temperature: 250°C
[0118] Carrier Gas: Helium
[0119] Detector: Hydrogen Flame Ionization Detector (FID)
[0120] In addition, the measurement conditions of the NMR spectra of the compounds isolated in Reference Examples and Examples are as follows.
[0121] Preparation of mixtures with compound and deuterated CDCl 3 (manufactured by Cambrige Isotope Laboratories, Inc., containing 0.05% TMS), using ...
reference example 1
[0123]
[0124] Bis(acetylacetonate)vanadium(IV) oxide (3.64 g, 20.6 mmol) was suspended in 2-propanol (100 mL), and 8-hydroxyquinoline (5.97 g, 41.1 mmol) was added. After stirring this at room temperature for 7 hours, the precipitated crystals were filtered and washed with 2-propanol (20 mL). The obtained wet crystals were dried under reduced pressure at 40° C. for 17 hours to obtain the vanadium complex (hereinafter referred to as “i-Pr complex”) represented by the above formula (2-1) (6.42 g, product rate of 75.2%).
[0125] 1 H-NMR (400MHz, CDCl 3 )δ8.60(s, 1H), 8.46(s, 1H), 8.09(d, 1H, J=8.0Hz), 8.02(d, 1H, J=8.4Hz), 7.51-7.56(m, 2H), 7.15-7.22(m, 6H), 6.26-6.32(m, 1H), 1.53(d, 3H, J=11.6Hz), 1.49(d, 3H, J=6.4Hz)
manufacture Embodiment 1
[0127]
[0128] Synthesis of the vanadium complex shown in
[0129] Bis(acetylacetonate)vanadium(IV) oxide (182 mg, 0.69 mmol) was suspended in acetonitrile (5 mL), and 8-hydroxyquinoline (199 mg, 1.37 mmol) and phenol (5 mL) were added thereto. This was stirred at room temperature for 6 hours, and the solvent was distilled off under reduced pressure. After adding diisopropyl ether (10 mL) to the residue, the precipitated crystals were filtered and washed with diisopropyl ether (5 mL). The obtained wet crystals were dried under reduced pressure at 40° C. for 7 hours to obtain the vanadium complex represented by the above formula (2a-1) (264 mg, yield 85.7%).
[0130] 1 H-NMR (400MHz, CDCl 3 )δ8.64(d, 1H, J=4.4Hz), 8.49(d, 1H, J=3.2Hz), 8.15(d, 1H, J=7.2Hz), 8.04(d, 1H, J=7.2Hz) , 7.54-7.60(m, 2H), 7.27-7.31(m, 6H), 7.17-7.23(m, 4H), 6.95-6.99(m, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com