Pig eperythrozoonosis vaccine and preparation method thereof
A technology of porcine eperythrozoon and porcine eperythrocytes, applied in biochemical equipment and methods, methods based on microorganisms, bacteria, etc., can solve the problems of no protective effect and little clinical significance, and achieve low cost and high production process And the effect of simple structure and good immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation of the vaccine adopts a more effective and convenient inactivation method, and its detection is also more intuitive and feasible.
[0019] The immune effect and protective effect of the vaccine can be tested through the combination of laboratory and clinical methods, which provides a reliable basis for future clinical application and improves the feasibility of the research.
Embodiment 1
[0020] Embodiment 1, the preparation of vaccine comprises the following steps:
[0021] 1. The cultivation of porcine Eperythrozoon. The erythrocytes infected with porcine Eperythrozoon were inoculated into cell culture flasks and placed at 37°C with 5% CO 2 After about 72 hours of culture in an incubator, the red blood cells hemolyzed and turned into ghosts with worms, and then cultured continuously for 30 days to fully reduce the components of the red blood cell membrane, and use ultrasonic waves to dissociate the ghosts of porcine eperythrocytic bodies into single pathogens, count them with a red blood cell counting board and continue After culture, the proliferation reached the peak at 48 hours, and the number of proliferation reached 30-40%, and then decreased. In the field of vision, 2-6 proliferating Eperythrozoon porcine can be seen, and then ultrasonicated into a single antigen for detection The number of them reaches 2.87x10 9 cells / mL for vaccine preparation. Med...
Embodiment 2
[0025] Embodiment 2, the evaluation of vaccine effect
[0026] 1. Safety evaluation.
[0027] The prepared vaccine was prepared as described above. The prepared vaccine was injected into 20 Kunming mice, each with 0.2 mL intramuscularly. The diet and mental state of the mice were observed. No significant changes were found, and no mice died at 21 days.
[0028] The prepared vaccine was injected into 10 sows, 10 suckling piglets, 10 nursery pigs, and 10 fattening pigs. The mental state, respiration, and pulse of the pigs were observed to be normal, and no death occurred in 21 days.
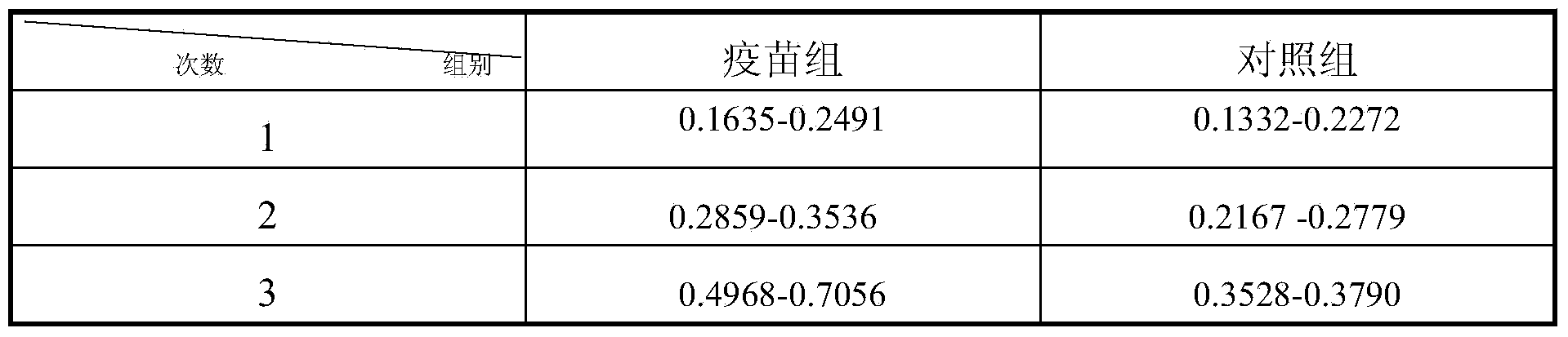
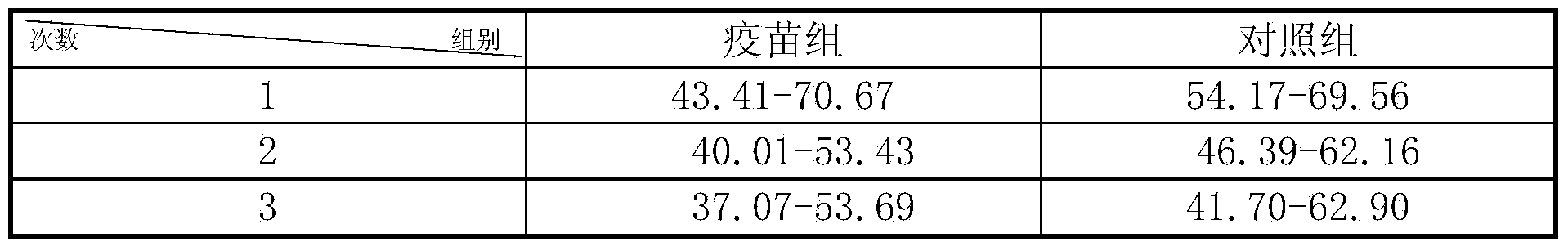
[0029] 2. Evaluation of immune effect in mice
[0030] Thirty Kunming mice were randomly divided into 2 groups, 15 in each group, injected intramuscularly with 0.2 mL of the prepared vaccine, and boosted once 7 days later. The control group was injected with the same dose of normal saline. The mental state of the mice in the control group and the immunized group was observed, and the mice were ki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com