Simple synthetic method of plant immunoactivator core fragment glucose trisaccharide
A technology of immune activation and core fragments, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
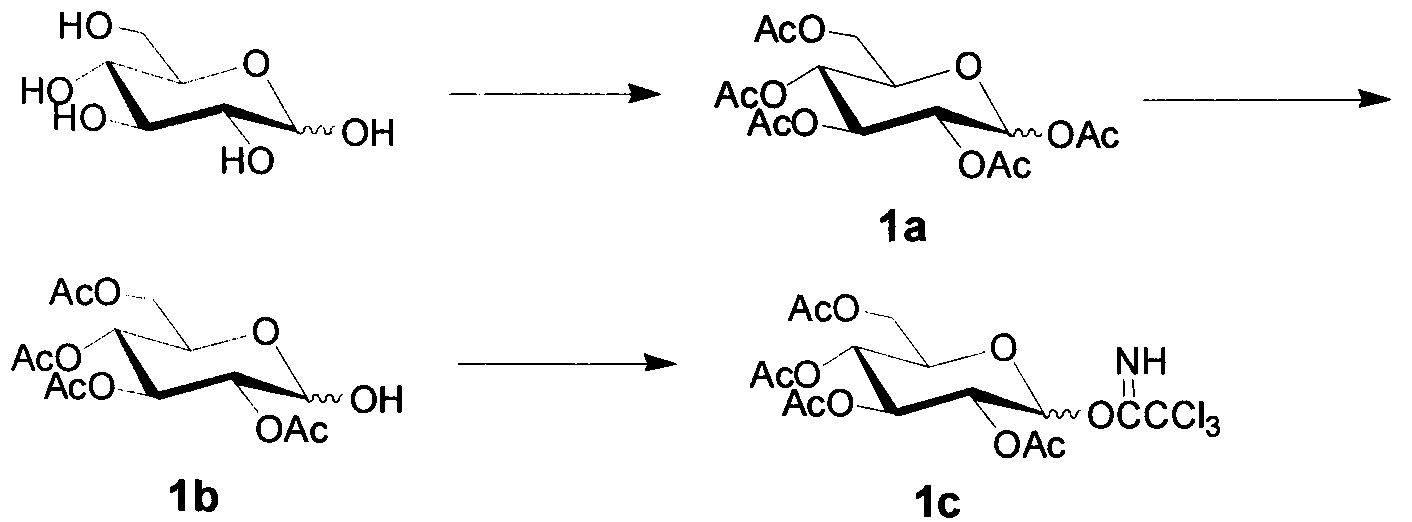
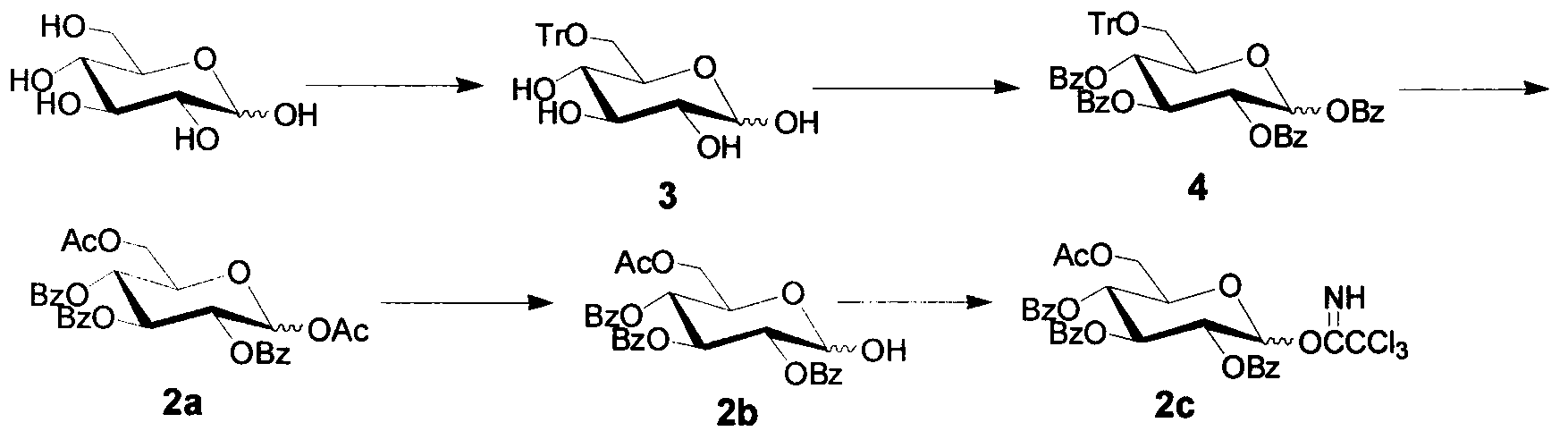
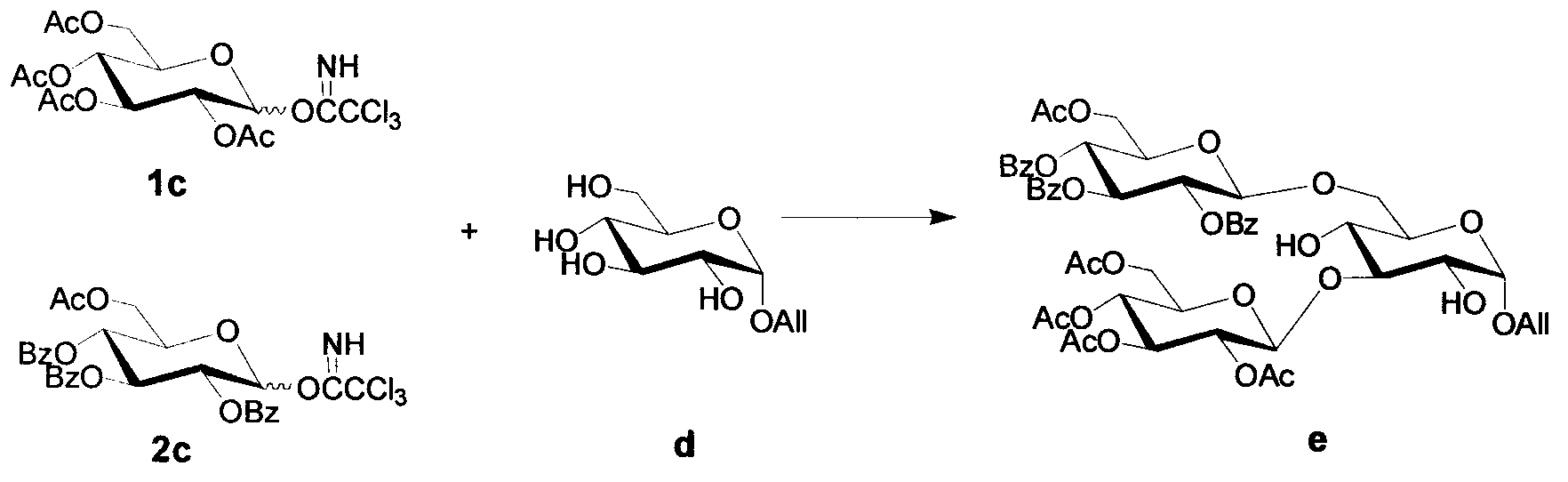
[0018] (1) Preparation of monosaccharide donor 1c
[0019] Measure Ac 2 O (450mL, 0.954mol) in a 1000mL three-neck flask, add HClO dropwise under stirring in an ice bath 4 (4.05mL, 0.05mol), the reaction solution changed from colorless to yellow, weighed dry glucose (95.1g, 0.528mol) and added it to the reaction system in batches, vigorously mechanically stirred under ice bath (control the reaction temperature not to exceed 20°C ), the reaction system gradually changed from yellow to yellow-green, and finally poured 450mL of ice water into the flask, continued to stir for 0.5 hours, and then added 500mL of CH to the reaction system 2 Cl 2 . Separation, water phase with CH 2 Cl 2 Extract (200mL×3), combine the organic phases, wash with water, and saturate with NaHCO 3 The solution was washed to neutrality, washed once with saturated NaCl solution, and the organic phase was washed with anhydrous NaCl 2 SO 4 After drying and suction filtration, the filtrate was concentrat...
Embodiment 2
[0029] (1) Preparation of monosaccharide donor 1c
[0030] Measure Ac 2 O (450mL, 0.954mol) in a 1000mL three-neck flask, add HClO dropwise under stirring in an ice bath 4(4.05mL, 0.05mol), the reaction solution changed from colorless to yellow, weighed dry glucose (95.1g, 0.528mol) and added it to the reaction system in batches, vigorously mechanically stirred under ice bath (control the reaction temperature not to exceed 20°C ), the reaction system gradually changed from yellow to yellow-green, and finally poured 450mL of ice water into the flask, continued to stir for 0.5 hours, and then added 500mL of CH to the reaction system 2 Cl 2 . Separation, water phase with CH 2 Cl 2 Extract (200mL×3), combine the organic phases, wash with water, and saturate with NaHCO 3 The solution was washed to neutrality, washed once with saturated NaCl solution, and the organic phase was washed with anhydrous NaCl 2 SO 4 After drying and suction filtration, the filtrate was concentrate...
Embodiment 3
[0040] (1) Preparation of monosaccharide donor 1c
[0041] Measure Ac 2 O (450mL, 0.954mol) in a 1000mL three-neck flask, add HClO dropwise under stirring in an ice bath 4 (4.05mL, 0.05mol), the reaction solution changed from colorless to yellow, weighed dry glucose (95.1g, 0.528mol) and added it to the reaction system in batches, vigorously mechanically stirred under ice bath (control the reaction temperature not to exceed 20°C ), the reaction system gradually changed from yellow to yellow-green, and finally poured 450mL of ice water into the flask, continued to stir for 0.5 hours, and then added 500mL of CH to the reaction system 2 Cl 2 . Separation, water phase with CH 2 Cl 2 Extract (200mL×3), combine the organic phases, wash with water, and saturate with NaHCO 3 The solution was washed to neutrality, washed once with saturated NaCl solution, and the organic phase was washed with anhydrous NaCl 2 SO 4 After drying and suction filtration, the filtrate was concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com