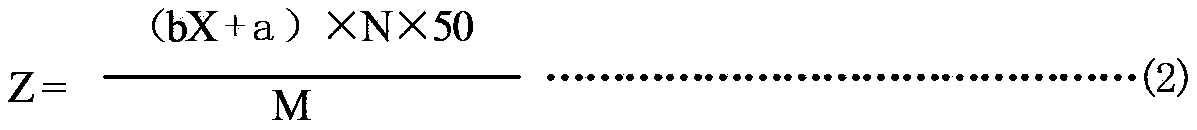Determination method for environmental cleaning performance of textile
A technology for environmental purification and measurement methods, applied in the direction of color/spectral characteristic measurement, etc., can solve problems such as quantitative evaluation of purification performance indicators, and achieve the effect of simple and easy test methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Nessler's reagent was prepared according to the following steps.
[0021] Weigh 12g of sodium hydroxide (NaOH) and dissolve it in 60ml of water.
[0022] Weigh 1.7g mercuric dichloride (HgCl 2 ) was dissolved in 30ml of water.
[0023] Weigh 3.5g of potassium iodide (KI) and dissolve in 10ml of water.
[0024] Under constant stirring, slowly add the mercuric dichloride solution into the potassium iodide solution until the red precipitate formed is no longer dissolved. Under constant stirring, slowly add the cold sodium hydroxide solution into the above mixed solution of mercuric dichloride and potassium iodide, then add the remaining mercuric dichloride solution, let it stand in the dark for 24 hours, extract the supernatant and store it In a brown bottle, stoppered tightly with a rubber stopper. Nessler's reagent needs to be kept refrigerated in the refrigerator and can be stored stably for one month.
[0025] 2. Sulfuric acid solution: ρ=1.84g / ml, c(H 2 SO 4...
Embodiment 2
[0059] 1. Nessler's reagent was prepared according to the following steps.
[0060] Weigh 12g of sodium hydroxide (NaOH) and dissolve it in 60ml of water.
[0061] Weigh 1.9g mercuric dichloride (HgCl 2 ) was dissolved in 30ml of water.
[0062] Weigh 4g of potassium iodide (KI) and dissolve in 12ml of water.
[0063] Under constant stirring, slowly add the mercuric dichloride solution into the potassium iodide solution until the red precipitate formed is no longer dissolved. Under constant stirring, slowly add the cold sodium hydroxide solution into the above mixed solution of mercuric dichloride and potassium iodide, then add the remaining mercuric dichloride solution, let it stand in the dark for 24 hours, extract the supernatant and store it In a brown bottle, stoppered tightly with a rubber stopper. Nessler's reagent needs to be kept refrigerated in the refrigerator and can be stored stably for one month.
[0064] 2. Sulfuric acid solution: ρ=1.84g / ml, c(H 2 SO 4 )=0...
Embodiment 3
[0067] The water used in the preparation of the Nessler's reagent is ammonia-free water.
[0068] Before weighing the weight of the textile sample, the textile sample was subjected to constant temperature and humidity treatment for more than 8 hours.
[0069] In the present invention, dilute sulfuric acid solution is used to extract the ammonia gas absorbed by the sample, and Nessler's reagent is used to react with ammonium ions in the extract to generate a yellow-brown complex. The chromaticity of the complex has a linear relationship with the concentration of ammonia. , Measure the absorbance at a wavelength of 420nm with a visible-ultraviolet spectrophotometer, and then calculate the concentration of ammonia in the extract and the ammonia absorption value of the sample. The test method is simple and accurate, and can quantitatively measure the purification performance of the fabric.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com