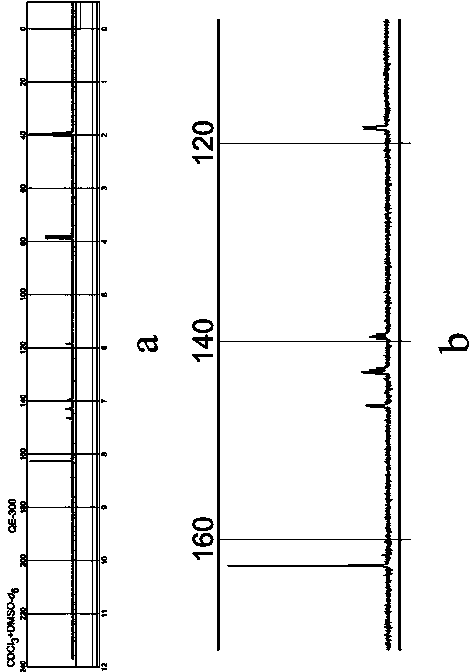
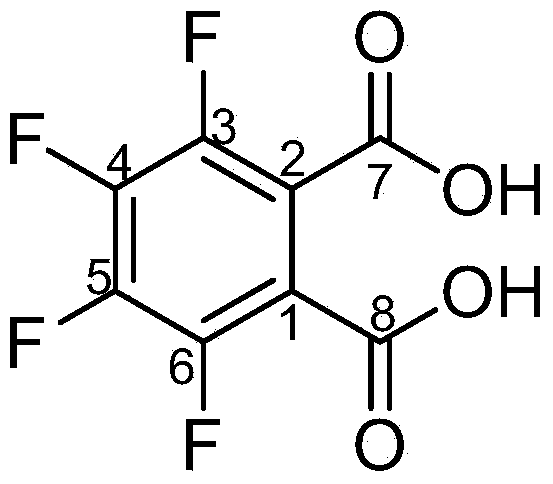
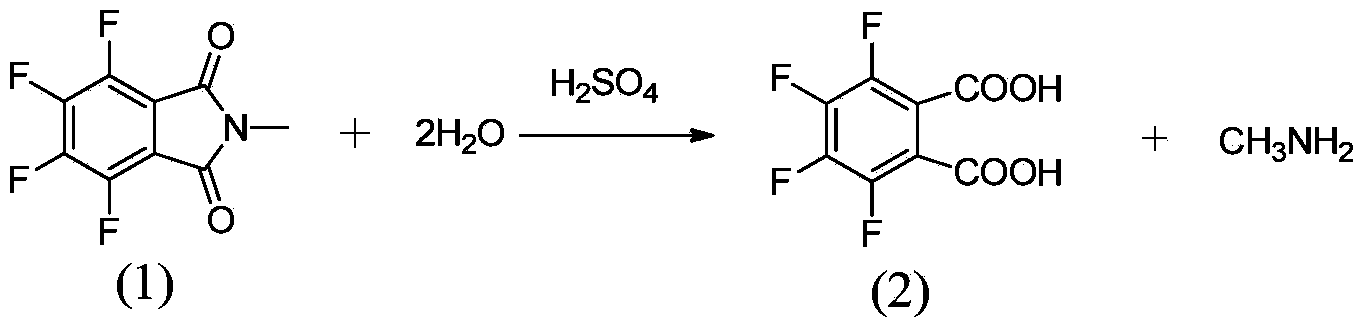
Method for preparing 3,4,5,6-tetrafluorophthalic acid
A technology of tetrafluorophthalic acid and methyltetrafluorophthalimide, which is applied in the field of green and clean 3,4,5,6-tetrafluorophthalic acid preparation, can solve the problems of restricting application, High cost, difficult wastewater treatment and other problems, to achieve the effect of reducing production costs, improving product yield, and reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 100 kilograms of N-methyltetrafluorophthalimide, 300 kilograms of water, and 0.8 kilograms of p-toluenesulfonic acid in the hydrolysis autoclave, at a pressure of 0.4kg / cm 2 , hydrolyzed at a temperature of 102°C, cooled to about 10°C after passing the central control, stirred and crystallized for 2 hours, centrifuged in a centrifuge, and dried the filter cake to obtain 3,4,5,6-tetrafluorophthalic acid. The rate is 80.7%.
Embodiment 2
[0021] Add 100 kilograms of N-methyltetrafluorophthalimide, 220 kilograms of water, and 0.8 kilograms of p-toluenesulfonic acid in the hydrolysis autoclave, at a pressure of 2.0kg / cm 2 , hydrolyzed at a temperature of 125°C, cooled to about 10°C after passing the central control, stirred and crystallized for 2 hours, centrifuged in a centrifuge, and dried the filter cake to obtain 3,4,5,6-tetrafluorophthalic acid. The rate is 93.5%.
Embodiment 3
[0023] Add 100 kilograms of N-methyltetrafluorophthalimide, 200 kilograms of water, and 0.8 kilograms of p-toluenesulfonic acid in the hydrolysis autoclave, at a pressure of 3.2kg / cm 2 , hydrolyzed at a temperature of 145°C, cooled to about 10°C after passing the central control, stirred and crystallized for 2 hours, centrifuged in a centrifuge, and dried the filter cake to obtain 3,4,5,6-tetrafluorophthalic acid. The rate is 79.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com