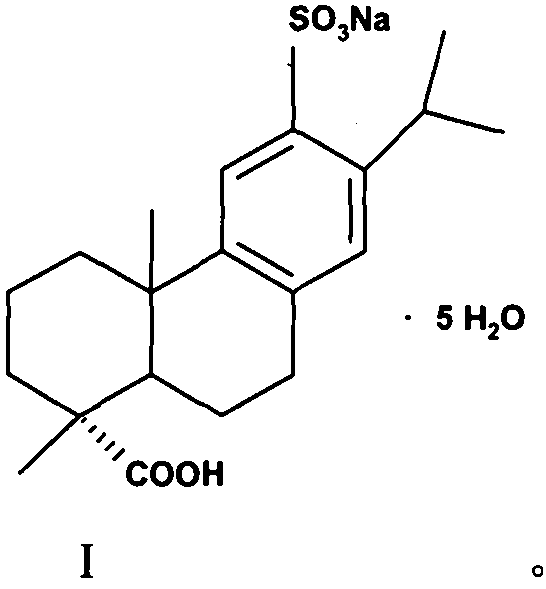
A kind of preparation method of ecabet sodium
A technology of icabet sodium and sodium salt is applied in the field of preparation of anti-peptic ulcer drug icabet sodium, which can solve the problems of low selectivity, low yield and the like, and achieves simple and easy-to-obtain raw materials and high yield. High and complete sulfonation conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
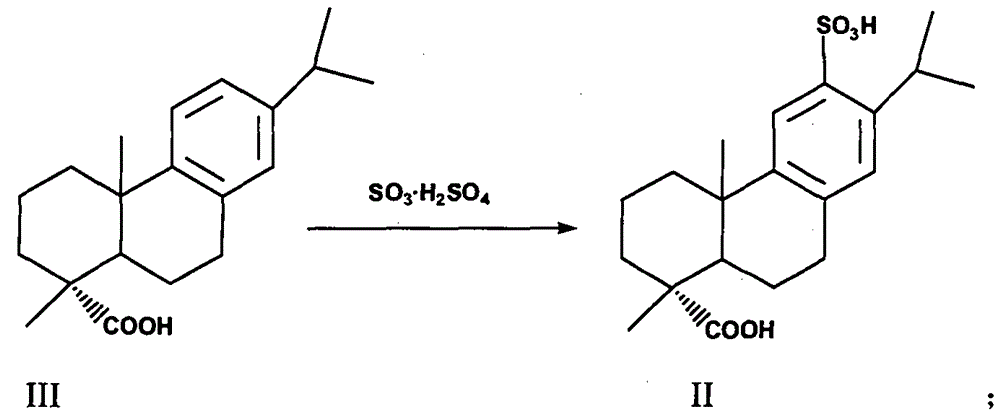
[0029] 1.1 Preparation of 12-sulfonic acid dehydroabietic acid (II)
[0030] Add compound (III) (100 g, 0.333 mol) into 20% oleum (399.6 g, containing 0.999 mol of sulfur trioxide) pre-cooled to 3°C to 10°C, stir and react for 1.5 to 2 hours, then pour into 1000 g of ice In water, a large amount of off-white precipitates were precipitated, filtered, and the filter cake was dried under reduced pressure to obtain compound (II) off-white solid (HPLC purity>99.0%, yield 91%).
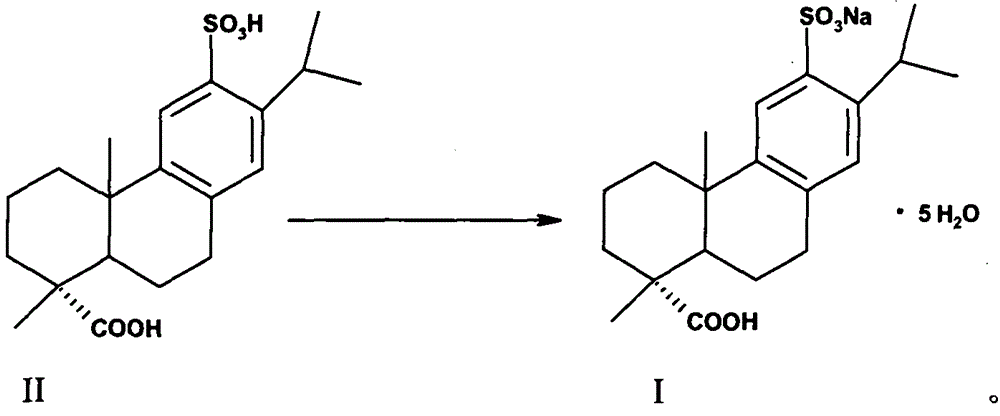
[0031] 1.2 Preparation of ecabet sodium (I)
[0032] Compound (II) (80g, 0.210mol) was added to an aqueous solution of 25% sodium isooctanoate (36.6g, 0.221mol) as a sodium forming agent, stirred and reacted for 0.5-1h, heated up, and filtered after dissolving and clarifying. Cool the filtrate in an ice-water bath to 0°C to 10°C, a large amount of white solids are precipitated, filter, and dry the filter cake under reduced pressure to obtain (I) white solid (specific rotation+72°, moisture 18.06%, HPLC pur...
Embodiment 2
[0034] 2.1 Preparation of 12-sulfonic acid dehydroabietic acid (II)
[0035] Add compound (III) (100 g, 0.333 mol) into 20% oleum (133.2 g, containing 0.333 mol of sulfur trioxide) pre-cooled to 3°C to 10°C, stir and react for 1.5 to 2 hours, then pour into 1000 g of ice In water, a large amount of off-white precipitates were precipitated, filtered, and the filter cake was dried under reduced pressure to obtain compound (II) off-white solid (HPLC purity>99.0%, yield 85%).
[0036] 2.2 Preparation of ecabet sodium (I)
[0037] Compound (II) (80 g, 0.210 mol) was added to an aqueous solution of sodium forming agent 25% sodium isooctanoate (17.55 g, 0.105 mol), stirred for 0.5 to 1 h, heated to raise the temperature, and filtered after being dissolved and clarified. Cool the filtrate in an ice-water bath to 0°C to 10°C, a large amount of white solids are precipitated, filter, and dry the filter cake under reduced pressure to obtain (1) white solid (specific rotation+70°, moistur...
Embodiment 3
[0039] 3.1 Preparation of 12-sulfonic acid dehydroabietic acid (II)
[0040] Add compound (III) (100g, 0.333mol) into 20% oleum (666g, containing 1.665mol of sulfur trioxide) pre-cooled to 3°C-10°C, stir for 1.5-2h, then pour into 1000g of ice water , a large amount of off-white precipitates were precipitated, filtered, and the filter cake was dried under reduced pressure to obtain compound (II) off-white solid (HPLC purity>98.0%, yield 88%).
[0041] 3.2 Preparation of ecabet sodium (I)
[0042]Compound (II) (80 g, 0.210 mol) was added to an aqueous solution of 25% sodium isooctanoate (70.2 g, 0.420 mol) as a sodium forming agent, stirred for 0.5 to 1 h, heated to raise the temperature, and filtered after being dissolved and clarified. Cool the filtrate in an ice-water bath to 0°C to 10°C, a large amount of white solids are precipitated, filter, and dry the filter cake under reduced pressure to obtain (1) white solid (specific rotation+71°, moisture 18.35%, HPLC purity>99.0%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com