Method for enhancing 13-valent pneumococcal polysaccharide protein bonder immunogenicity
A pneumococcal polysaccharide, immunogenic technology, applied in the direction of carrier-binding antigen/hapten components, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of immunogenic differences, protective limitations, and large immunogenicity variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The following examples illustrate specific implementation methods of the present invention, but are not limited to the following examples.
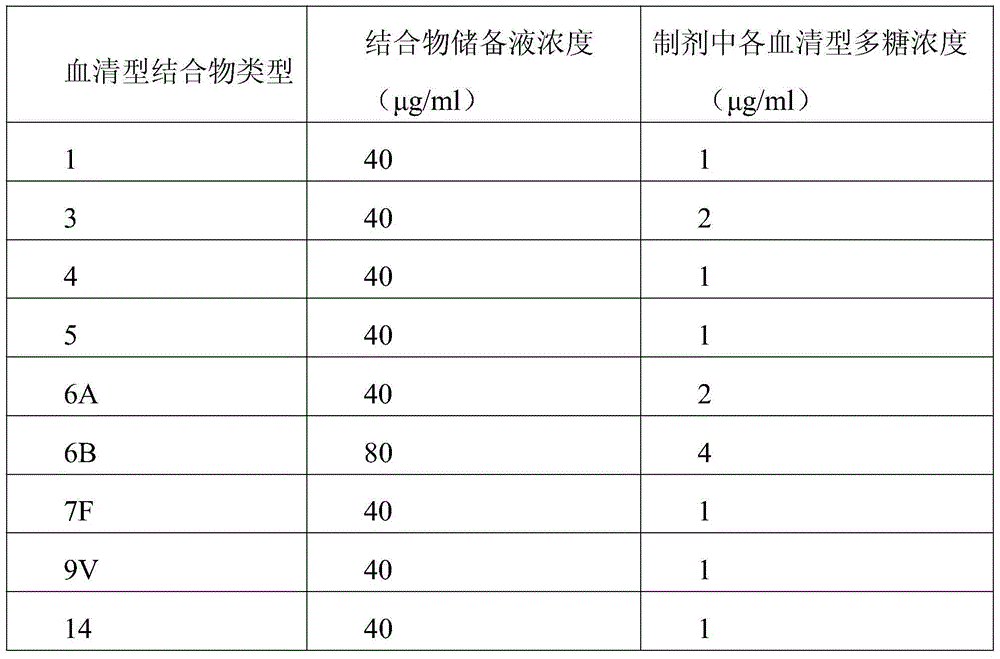
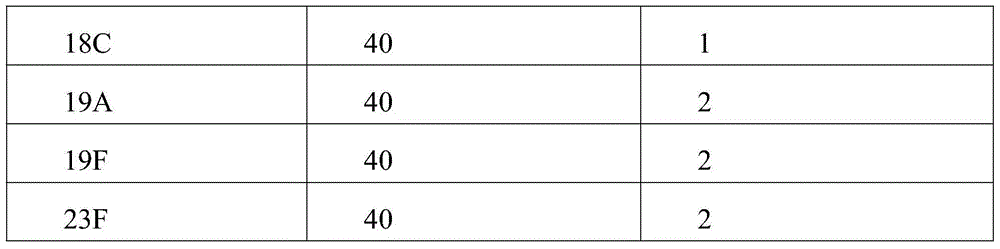
[0032] First step, preparation of carrier protein and pneumococcal polysaccharide
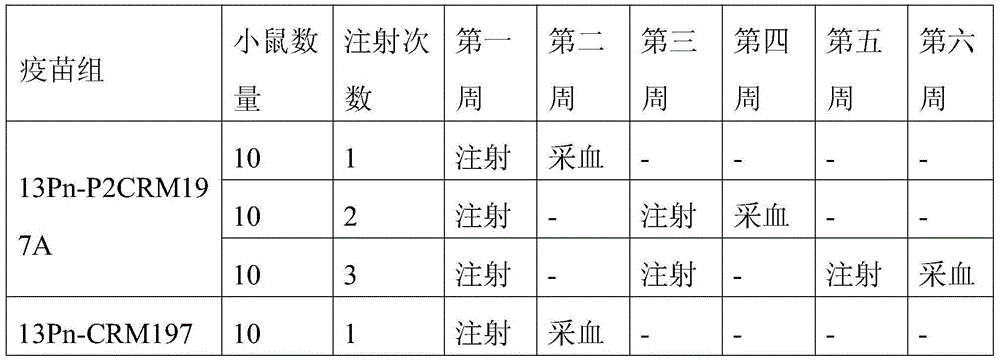
[0033] In order to demonstrate the effectiveness of the present invention, two carrier proteins were prepared, namely the protein carrier P2CRM197A containing P2 and the protein carrier CRM197A not containing P2. Among them, the CRM197A protein carrier is used to synthesize the 13-valent pneumococcal polysaccharide-CRM197A conjugate sample for control.
[0034] 1. Design of amino acid sequences of CRM197A protein carrier and P2CRM197A protein carrier
[0035] 1. Design of amino acid sequence of CRM197A protein carrier
[0036] Diphtheria toxin is expressed in Bacillus diphtheriae by β phage with diphtheria toxin gene, and exists in the form of polypeptide in bacterial cytoplasm, which is composed of 560 amino acids and has a molecular weight of 62...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com