Method for improving immunogenicity of 13-valent pneumococcal polysaccharide-protein conjugate
A pneumococcal polysaccharide and immunogenicity technology, applied in the direction of carrier-binding antigen/hapten components, antibacterial drugs, drug combinations, etc., can solve problems such as poor immune effect, large variability of immunogenicity, and differences in immunogens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The specific implementation method of the present invention is illustrated below, but not limited to the following examples.
[0032] The first step, preparation of carrier protein and pneumococcal polysaccharide
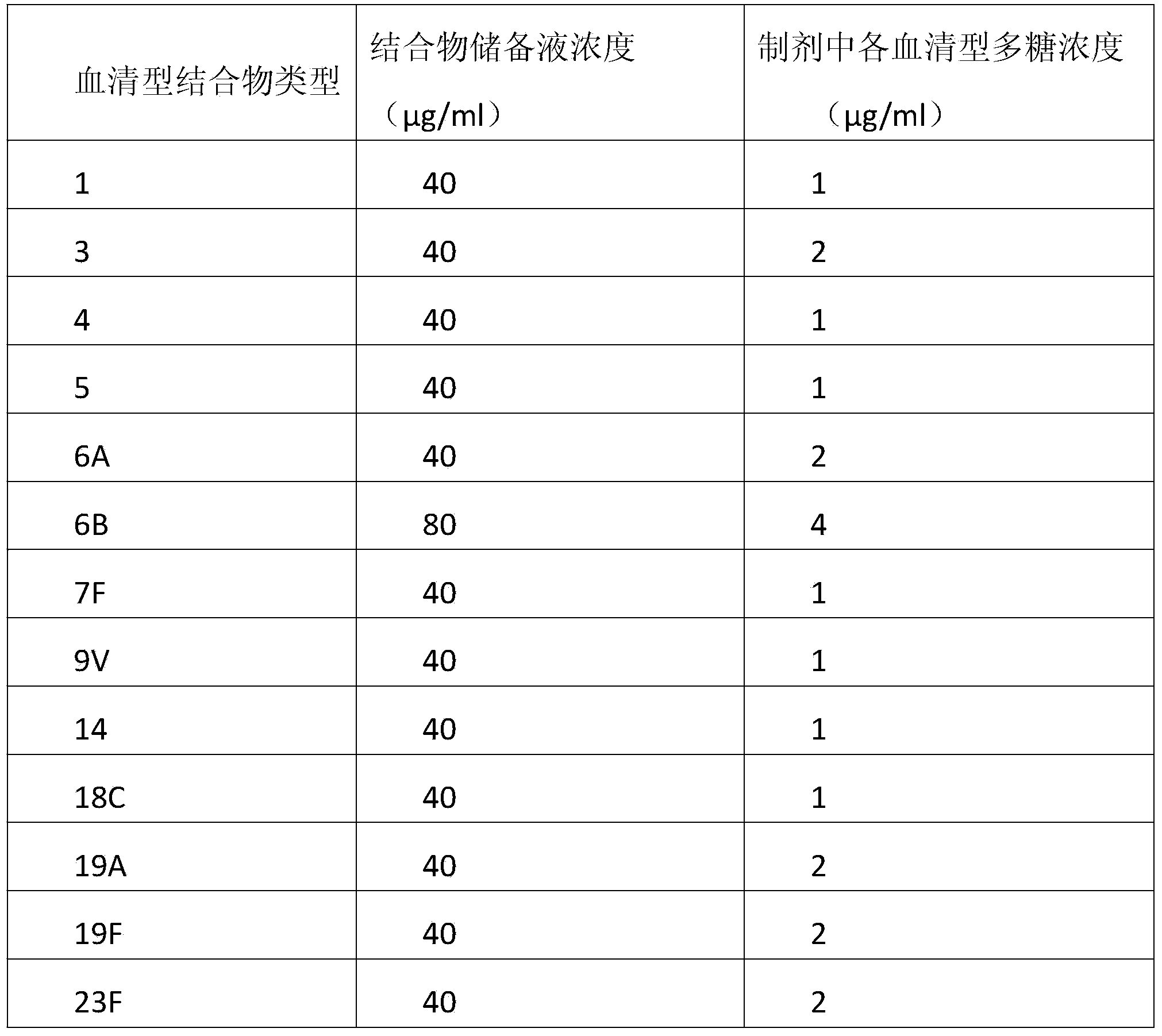
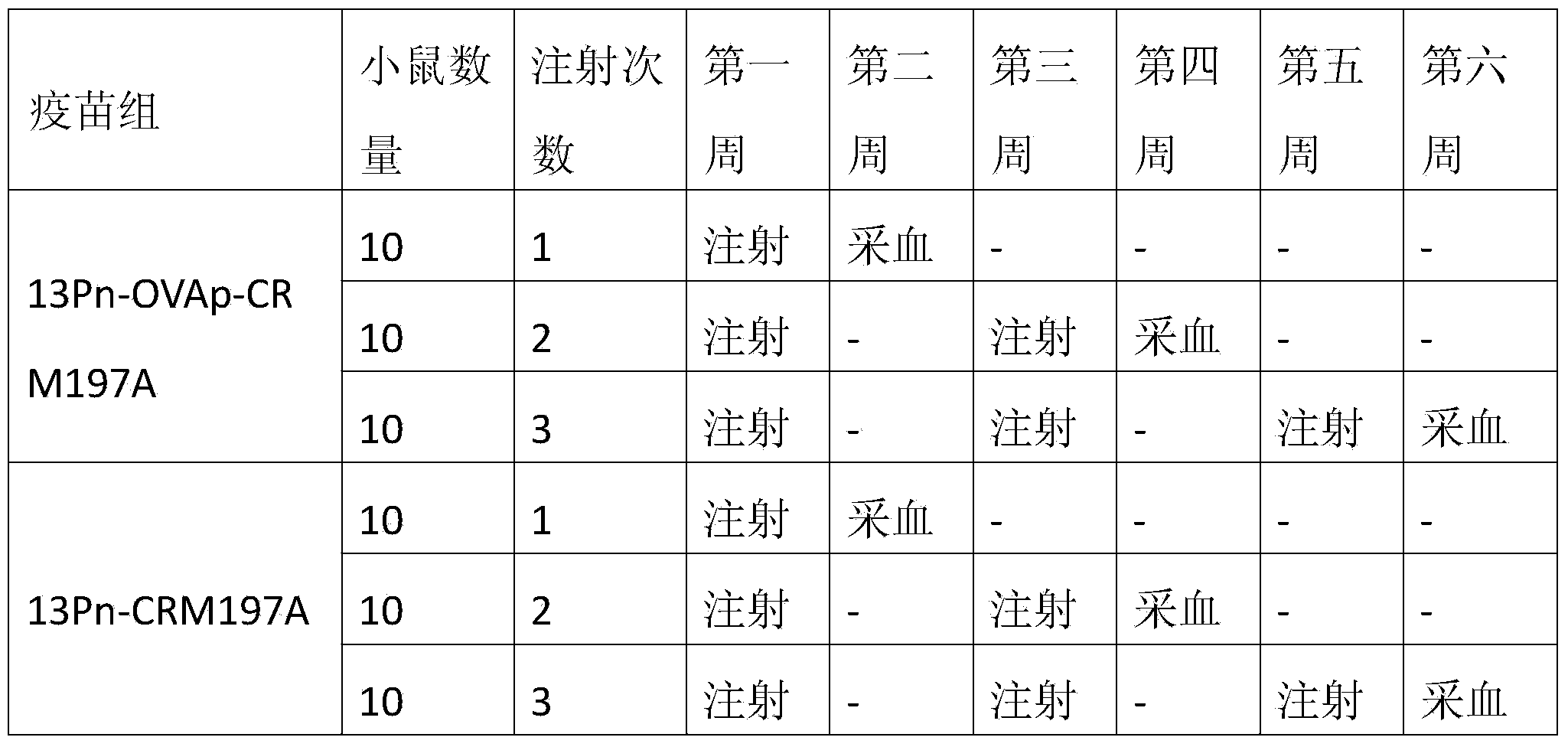
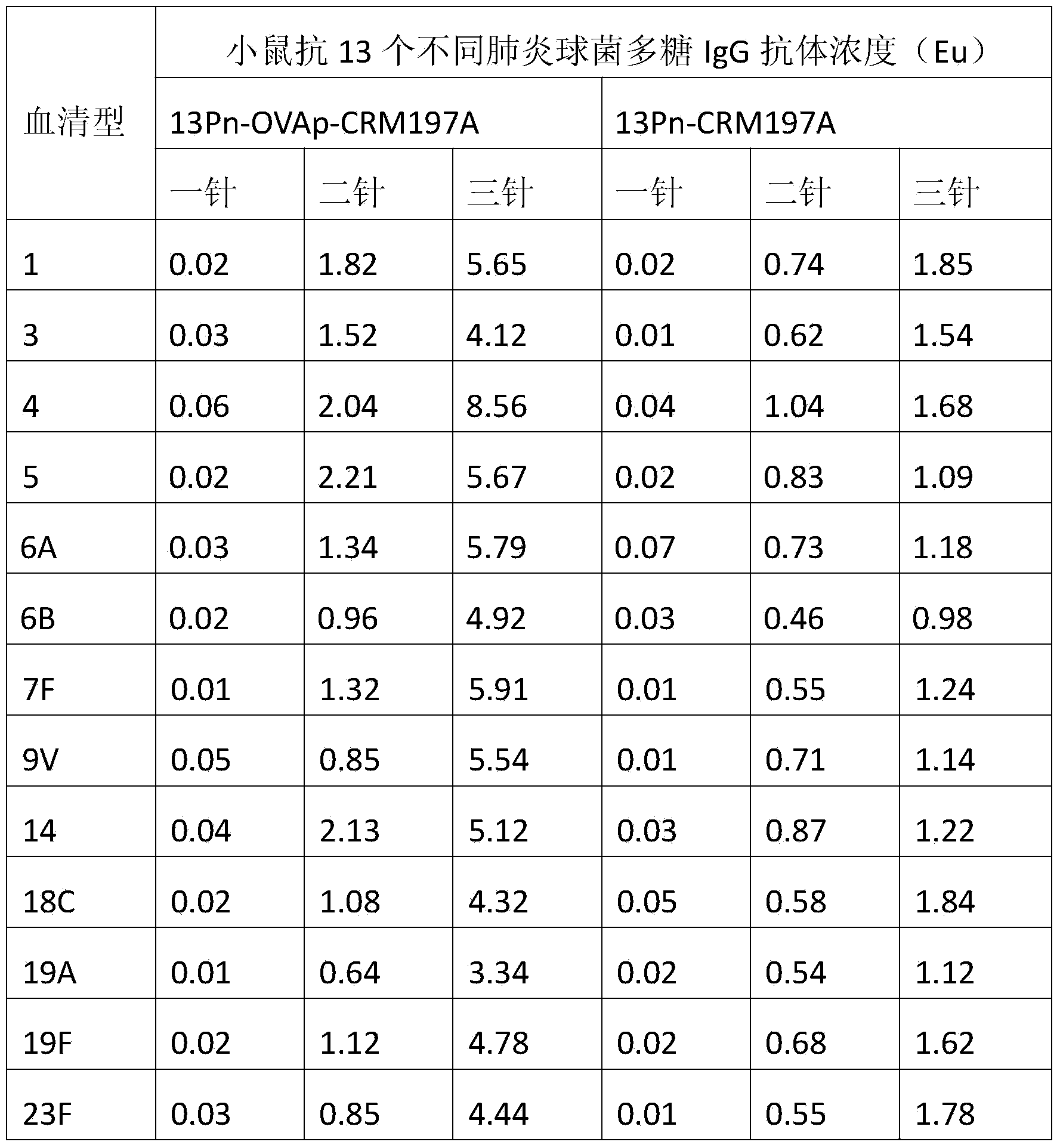
[0033] In order to illustrate the effectiveness of the present invention, two carrier proteins were prepared, namely the protein carrier OVApCRM197A containing OVAp and the protein carrier CRM197A not containing OVAp. Among them, the CRM197A protein carrier is used to synthesize the 13-valent pneumococcal polysaccharide-CRM197A conjugate sample for control.
[0034] 1. Design of amino acid sequence of CRM197A protein carrier and OVApCRM197A protein carrier
[0035] 1. Design of amino acid sequence of CRM197A protein carrier
[0036] Diphtheria toxin is expressed in Bacillus diphtheriae by β-phage with diphtheria toxin gene, and exists in the form of polypeptide in the bacterial cytoplasm, consisting of 560 amino acids with a molecular weight of 62,000 Dalto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com