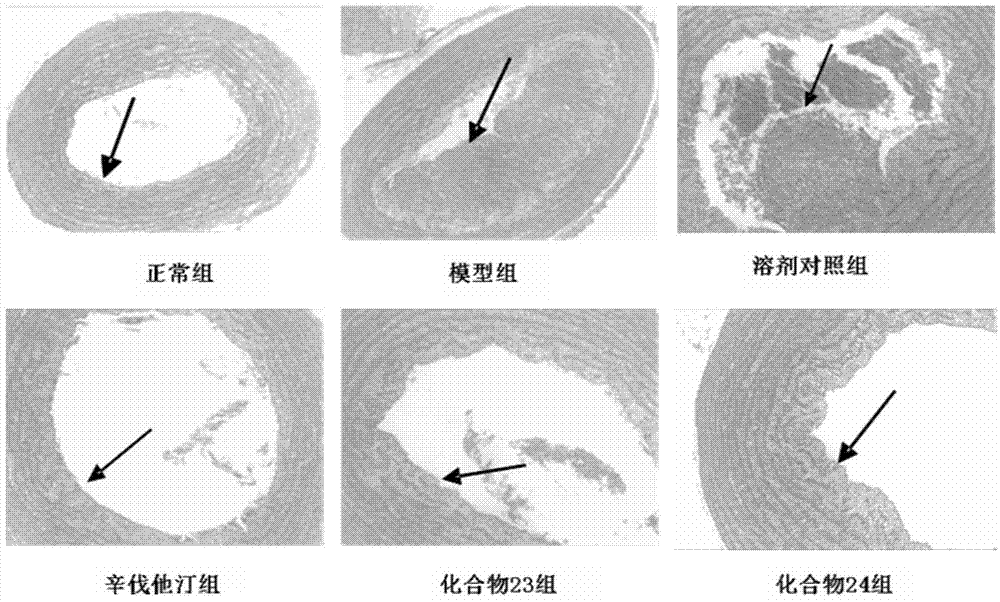
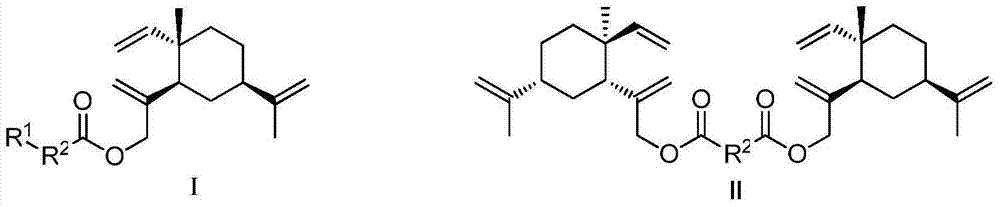
Beta-elemene 14-site ramification and application of beta-elemene 14-site ramification in treating atherosclerosis
A kind of technology of elemenol ester and compound, which is applied in the field of preparation of β-elemene 14-position derivatives and β-elemene 14-position derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
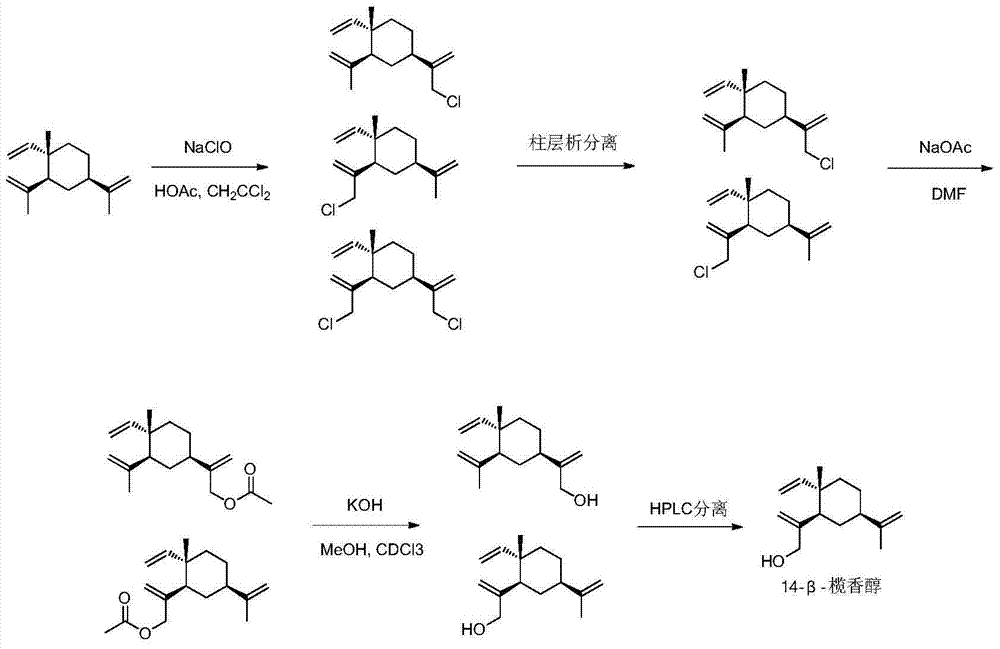
[0074] The preparation method of intermediate 14-beta-elemenol (compound 1)
[0075] Dissolve 100mmol of β-elemene in 20mL of dichloromethane and acetic acid mixed solution (V:V=2:1), slowly drop into sodium hypochlorite solution containing 180mmol of active chlorine in ice bath, and react in ice bath for 4h. The dichloromethane layer was separated, the aqueous layer was extracted three times with dichloromethane, the combined dichloromethane was concentrated to obtain a light yellow liquid crude product, without further purification, the liquid crude product was dissolved in 15 mL of anhydrous N,N-dimethylformaldehyde Add 200mmol of anhydrous sodium acetate under stirring to amide (DMF), and react at 100°C for 7h. The reaction solution was suction-filtered with diatomaceous earth, and the filtrate was added with 15 mL of saturated brine, and extracted three times with petroleum ether. The petroleum ether was concentrated to obtain a yellow liquid, which was separated by colu...
Embodiment 2
[0079] Preparation method of 4-(14-beta-elemeneoxy)-4-oxobutanoic acid (compound 2)
[0080] Dissolve 3mmol of 14-β-elemenol in 10mL of anhydrous dichloromethane, add 0.3mmol of DMAP, 0.3mmol of EDCI and 3.3mmol of succinic anhydride, and react at room temperature for 10h. The reaction solution was washed three times with 10% hydrochloric acid, and the dichloromethane layer was concentrated to obtain a pale yellow liquid. Column chromatography with petroleum ether: ethyl acetate = 8:1 (V:V) gave a colorless liquid product with a yield of 67%.
[0081] 1 H NMR (CDCl 3 ,300MHz)δ:0.99(s,3H),1.40-1.65(m,6H),1.74(s,3H),1.92-1.97(m,1H),2.01-2.06(m,1H),2.63-2.73( m,4H),4.51(s,2H),4.72(s,2H),4.89-4.95(m,3H),5.13(s,1H),5.75(dd,J 1 =17.8Hz,J 2 =10.4Hz,1H).
[0082]13 C NMR (CDCl 3 ,300MHz)δ:177.9,171.7,150.0,149.3,145.9,113.6,111.0,108.4,68.6,48.3,45.6,39.7,33.0,28.9,26.7,21.0,16.0.
Embodiment 3
[0084] Preparation method of 5-(14-beta-elemenyloxy)-5-oxopentanoic acid (compound 3)
[0085] Dissolve 3mmol of 14-β-elemenol in 10mL of anhydrous dichloromethane, add 0.3mmol of DMAP, 0.3mmol of EDCI and 3.3mmol of glutaric anhydride, and react at room temperature for 10h. The reaction solution was washed three times with 10% hydrochloric acid, and the dichloromethane layer was concentrated to obtain a light yellow liquid. Column chromatography with petroleum ether: ethyl acetate = 8:1 (V:V) gave a colorless liquid product with a yield of 74%.
[0086] 1 H NMR (CDCl 3 ,300MHz)δ:1.00(s,3H),1.41-1.65(m,6H),1.74(s,3H),1.92-2.06(m,4H),2.44(t,J=7.3Hz,4H),4.49 (s,2H),4.71(s,2H),4.88(s,1H),4.90(d,J=4.7Hz,1H),4.95(s,1H),5.13(s,1H),5.75(dd, J 1 =17.8Hz,J 2 =10.4Hz,1H).
[0087] 13 C NMR (CDCl 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com