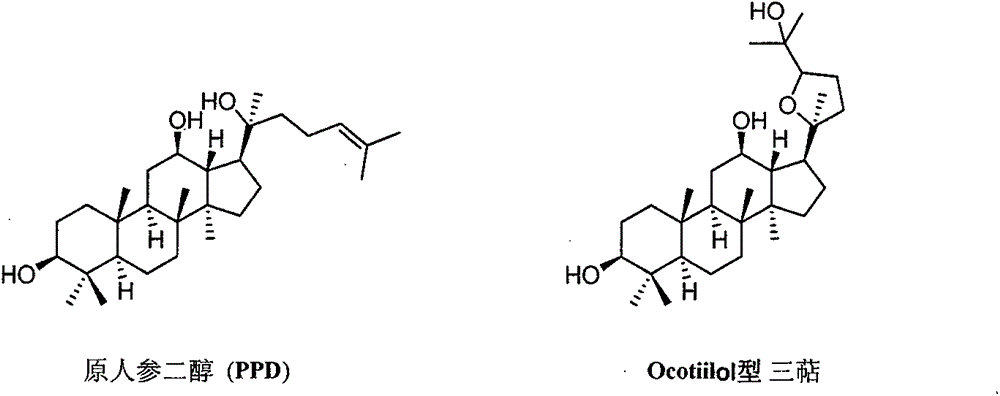
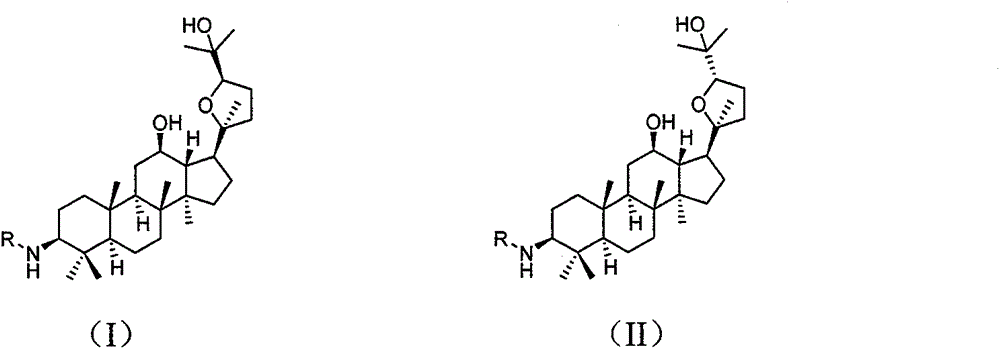
Preparation method and use of (20S, 24R/S)-epoxy-12 beta, 25-hydroxy-dammarane-3 beta-amine derivatives
An epoxy and drug technology, used in medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, which can solve problems such as threats to human health, reduced sensitivity, and drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (20S, 24S)-epoxydammarane-12β, 25-diol-3β-amine (OSA)
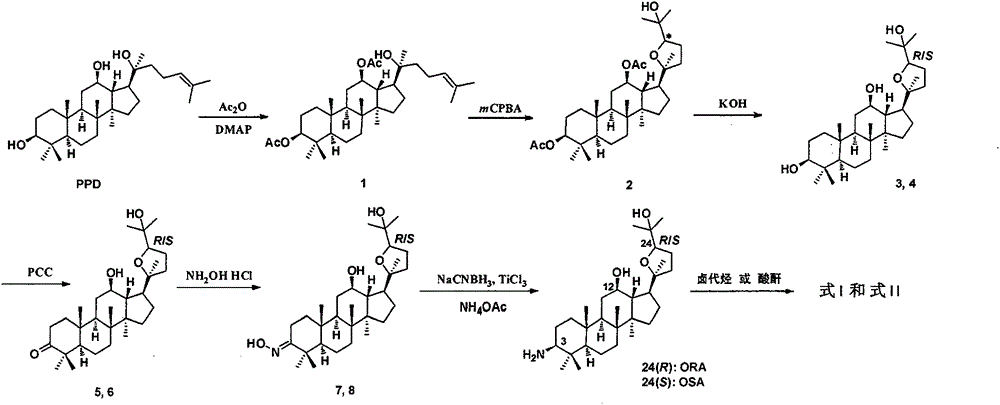
[0052] Dissolve 20(S)-protopanaxadiol (500mg, 1.08mmol) in anhydrous pyridine (3mL), add DMAP (20mg, 0.16mmol), stir well and slowly add acetic anhydride (0.42mL, 4.43mmol) dropwise , stirred at room temperature for 12h. Dilute with ethyl acetate (20 mL), wash with 10% hydrochloric acid until acidic, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography (petroleum ether: ethyl acetate = 10:1) to give white Solid 1 (508 mg, 85%).
[0053]The above-obtained 1 (208 mg, 0.38 mmol) was dissolved in anhydrous dichloromethane (6 mL), and the dichloromethane of m-chloroperoxybenzoic acid (185 mg, 75%, 0.16 mmol) was slowly added dropwise under ice-salt bath precooling. Methane (5 mL) solution, half an hour after the dropwise addition was completed, the mixture was raised to room temperature and stirred for 2 h. Add isopropanol (0.1mL), continue to s...
Embodiment 2
[0061] (20S, 24R)-epoxydammarane-12β, 25-diol-3β-amine (ORA)
[0062] Referring to the synthesis method of (20S, 24S)-epoxydammarane-12β, 25-diol-3-amine (OSA), a white solid ORA (120 mg, 73%) was obtained from compound 4 through compounds 6 and 8. 1 H NMR (CDCl 3 , 300MHz) δ3.84(dd, J=13.7Hz, 7.0Hz, 1H), 3.50(td, J=10.4Hz, 4.6Hz, 1H), 2.35(dd, J=11.5Hz, 4.4Hz, 1H), 2.18(td, J=10.9Hz, 3.5Hz, 1H), 1.28(s, 3H), 1.26(s, 3H), 1.09(s, 3H), 0.98(s, 3H), 0.94(s, 3H), 0.90(s, 3H), 0.83(s, 6H), 0.74(s, 3H). 13 C NMR (CDCl 3 , 75MHz) δ86.5, 85.4, 80.0, 70.1, 59.7, 56.6, 52.0, 50.6, 49.4, 47.9, 39.6, 39.5, 38.1, 37.3, 34.8, 32.6, 31.2, 28.6, 28.3, 27.9, 27.6, 26.1, 25.0 , 18.6, 18.1, 16.2, 15.5, 15.4; ESI-MS m / z 476.4 [M+H] + ; HR-MS (ESI) m / z: calculated for C 30 h 54 NO 3 [M+H] + : 476.4098, found: 476.4091.
Embodiment 3
[0064] (20S, 24S)-epoxy-3β-methylaminodammarane-12β, 25-diol
[0065] (20S,24S)-epoxydammarane-12β,25-diol-3-amine (OSA, 200 mg, 0.42 mmol) and anhydrous potassium carbonate (60 mg, 0.42 mmol) were added to anhydrous THF (8 mL) , then iodomethane (60 mg, 0.42 mmol) was added. N 2 React at room temperature for 5 hours under protection. Diluted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and column chromatographed to give a pale yellow product (155 mg, 75%). 1 H NMR (CDCl 3 , 300MHz) δ3.86(dd, J=10.2Hz, 5.0Hz, 1H), 3.51(td, J=9.8Hz, 4.0Hz, 1H), 3.16(s, 3H), 2.54(m, 1H), 2.22 (td, J=9.8Hz, 5.8Hz, 1H), 1.26(s, 3H), 1.21(s, 3H), 1.10(s, 3H), 1.02(s, 3H), 1.00(s, 3H), 0.89 (s,6H), 0.88(s,3H), 0.85(s,3H); ESI-MS m / z 490.4[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com