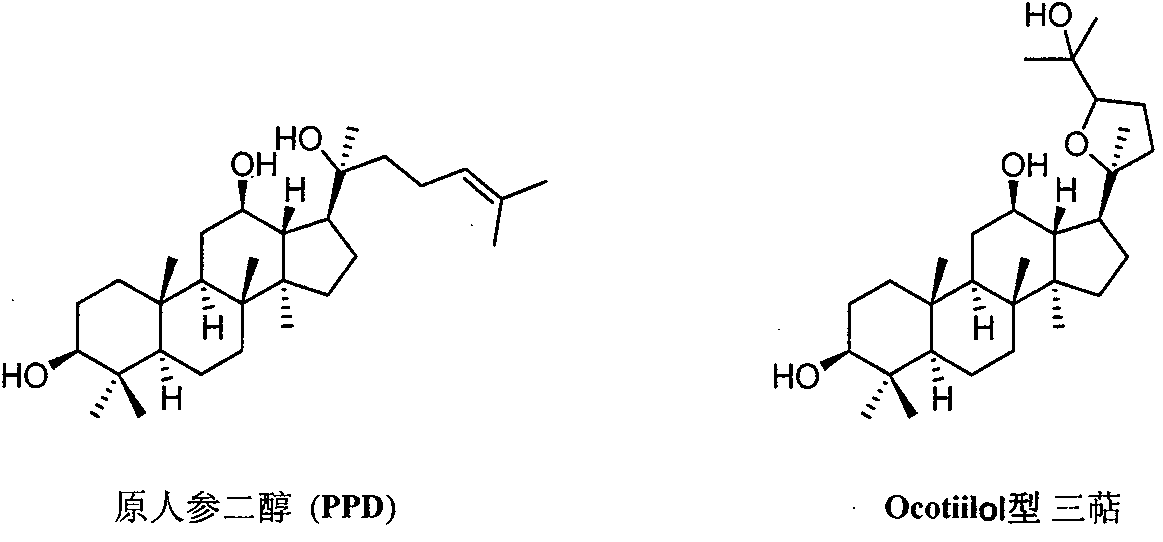
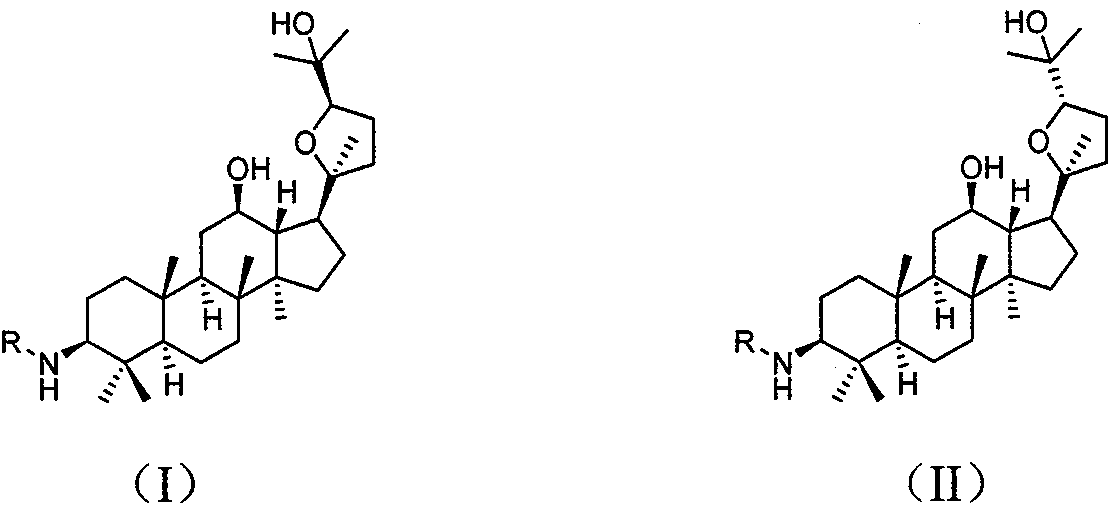
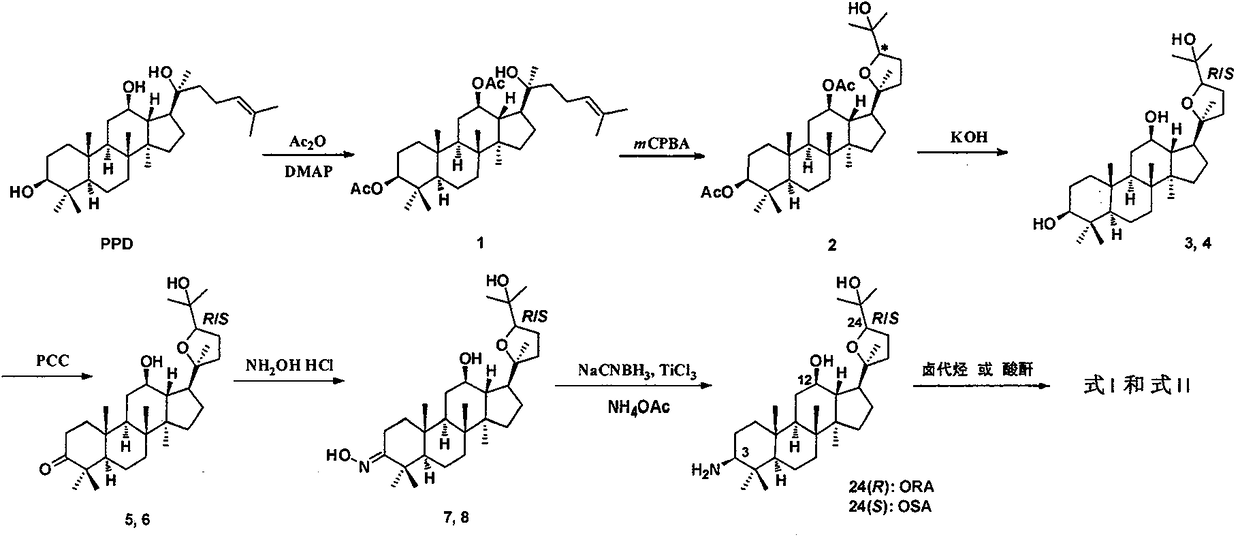
Preparation method and application of (20s, 24r/s)-epoxy-12β, 25-hydroxyl-dammarane-3β-amine derivative
A technology of epoxydammarane and drugs, which is applied in the application field of preparing drugs for reversing multi-drug resistance of tumors, and can solve problems such as threats to human health, drug resistance, and sensitivity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (20S, 24S)-epoxydammarane-12β, 25-diol-3β-amine (OSA)
[0052] Dissolve 20(S)-protopanaxadiol (500mg, 1.08mmol) in anhydrous pyridine (3mL), add DMAP (20mg, 0.16mmol), stir well and slowly add acetic anhydride (0.42mL, 4.43mmol) dropwise , stirred at room temperature for 12h. Dilute with ethyl acetate (20 mL), wash with 10% hydrochloric acid until acidic, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography (petroleum ether: ethyl acetate = 10:1) to give white Solid 1 (508 mg, 85%).
[0053]The above-obtained 1 (208 mg, 0.38 mmol) was dissolved in anhydrous dichloromethane (6 mL), and the dichloromethane of m-chloroperoxybenzoic acid (185 mg, 75%, 0.16 mmol) was slowly added dropwise under ice-salt bath precooling. Methane (5 mL) solution, half an hour after the dropwise addition was completed, the mixture was raised to room temperature and stirred for 2 h. Add isopropanol (0.1mL), continue to s...
Embodiment 2
[0061] (20S, 24R)-epoxydammarane-12β, 25-diol-3β-amine (ORA)
[0062] Referring to the synthesis method of (20S, 24S)-epoxydammarane-12β, 25-diol-3-amine (OSA), a white solid ORA (120 mg, 73%) was obtained from compound 4 through compounds 6 and 8. 1 H NMR (CDCl 3 , 300MHz) δ3.84(dd, J=13.7Hz, 7.0Hz, 1H), 3.50(td, J=10.4Hz, 4.6Hz, 1H), 2.35(dd, J=11.5Hz, 4.4Hz, 1H), 2.18(td, J=10.9Hz, 3.5Hz, 1H), 1.28(s, 3H), 1.26(s, 3H), 1.09(s, 3H), 0.98(s, 3H), 0.94(s, 3H), 0.90(s, 3H), 0.83(s, 6H), 0.74(s, 3H). 13 C NMR (CDCl 3 , 75MHz) δ86.5, 85.4, 80.0, 70.1, 59.7, 56.6, 52.0, 50.6, 49.4, 47.9, 39.6, 39.5, 38.1, 37.3, 34.8, 32.6, 31.2, 28.6, 28.3, 27.9, 27.6, 26.1, 25.0 , 18.6, 18.1, 16.2, 15.5, 15.4; ESI-MS m / z 476.4 [M+H] + ; HR-MS (ESI) m / z: calculated for C 30 h 54 NO 3 [M+H] + : 476.4098, found: 476.4091.
Embodiment 3
[0064] (20S, 24S)-epoxy-3β-methylaminodammarane-12β, 25-diol
[0065] (20S,24S)-epoxydammarane-12β,25-diol-3-amine (OSA, 200 mg, 0.42 mmol) and anhydrous potassium carbonate (60 mg, 0.42 mmol) were added to anhydrous THF (8 mL) , then iodomethane (60 mg, 0.42 mmol) was added. N 2 React at room temperature for 5 hours under protection. Diluted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and column chromatographed to give a pale yellow product (155 mg, 75%). 1 H NMR (CDCl 3 , 300MHz) δ3.86(dd, J=10.2Hz, 5.0Hz, 1H), 3.51(td, J=9.8Hz, 4.0Hz, 1H), 3.16(s, 3H), 2.54(m, 1H), 2.22 (td, J=9.8Hz, 5.8Hz, 1H), 1.26(s, 3H), 1.21(s, 3H), 1.10(s, 3H), 1.02(s, 3H), 1.00(s, 3H), 0.89 (s,6H), 0.88(s,3H), 0.85(s,3H); ESI-MS m / z 490.4[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com