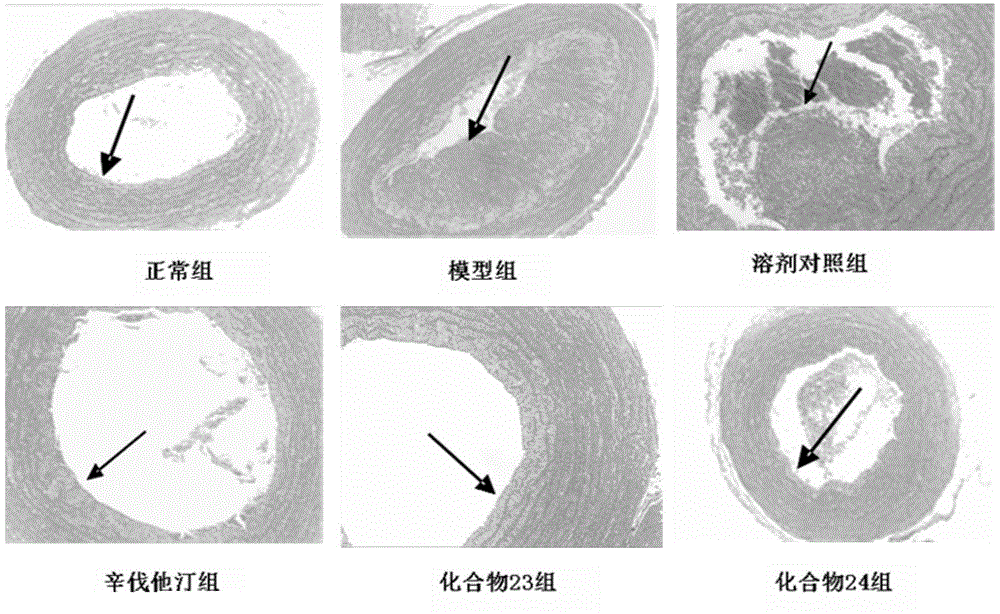
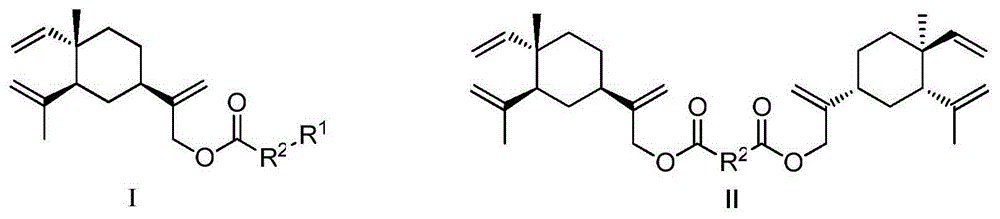
Beta-elemene 13-site derivative and use thereof in treatment of atherosclerosis
A technology of elemyl esters and compounds, applied in the fields of organic synthesis and medicinal chemistry, can solve the problems of no reports of antioxidant activity derivatives, low bioavailability, and single structural modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
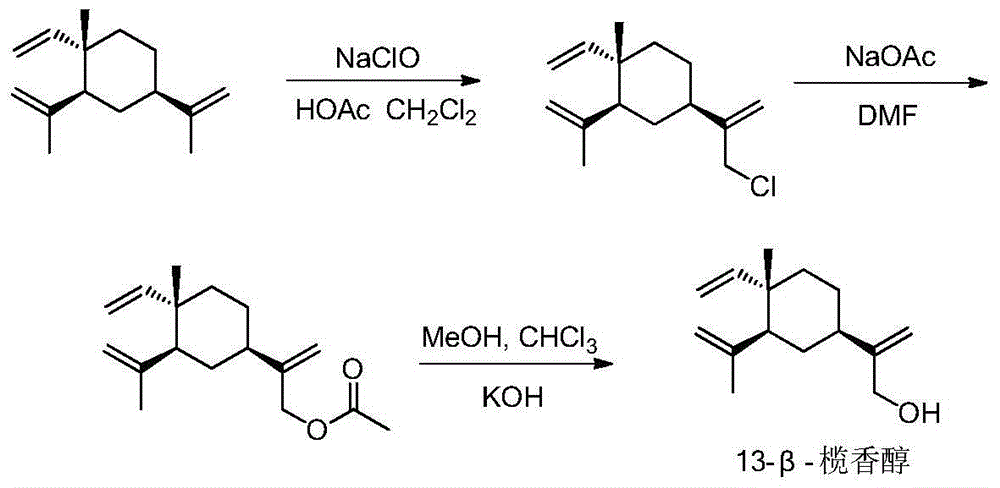
[0073] The preparation method of intermediate 13-beta-elemenol (compound 1)
[0074] Dissolve 100mmol of β-elemene in 20mL of dichloromethane and acetic acid mixed solution (V:V=2:1), slowly drop into sodium hypochlorite solution containing 180mmol of active chlorine in ice bath, and react in ice bath for 4h. The dichloromethane layer was separated, the aqueous layer was extracted 3 times with dichloromethane, the combined dichloromethane was concentrated to obtain a light yellow liquid crude product, the liquid crude product was dissolved in 15 mL of anhydrous N,N-dimethylformamide (DMF), stirred Add 200mmol of anhydrous sodium acetate at 100°C for 7h. The reaction solution was suction-filtered with 200-mesh silica gel, and the filtrate was added with 15 mL of saturated saline, and extracted three times with petroleum ether. Concentrate petroleum ether to obtain a yellow liquid, which is dissolved in a mixed solution of 8 mL methanol and 8 mL chloroform, and 200 mmol potassi...
Embodiment 2
[0078] Preparation method of 4-(13-beta-elemenyloxy)-4-oxobutanoic acid (compound 2)
[0079] Dissolve 3mmol 13-β-elemenol in 10mL of anhydrous dichloromethane, add 0.3mmol 4-dimethylaminopyridine (DMAP), 0.3mmol 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (EDCI) and 3.3 mmol succinic anhydride were reacted at room temperature for 10 h. The reaction solution was washed three times with 10% hydrochloric acid, and the dichloromethane layer was concentrated to obtain a light yellow liquid. Column chromatography with petroleum ether: ethyl acetate = 8:1 (V:V) gave a colorless liquid product with a yield of 60%.
[0080] 1 H NMR (CDCl 3 ,300MHz)δ:1.01(s,3H),1.41-1.68(m,6H),1.71(s,3H),1.99-2.04(m,2H),2.64-2.74(m,4H),4.59(s, 1H), 4.62(s, 2H), 4.83(s, 1H), 4.88(s, 1H), 4.93(d, J=4.0Hz, 1H), 5.01(s, 1H), 5.05(s, 1H), 5.81(dd,J 1 =17.8Hz,J 2 =10.5Hz,1H).
[0081] 13 C NMR (CDCl 3 .
Embodiment 3
[0083] Preparation method of 5-(13-beta-elemenyloxy)-5-oxopentanoic acid (compound 3)
[0084] Dissolve 3mmol of 13-β-elemenol in 10mL of anhydrous dichloromethane, add 0.3mmol of DMAP, 0.3mmol of EDCI and 3.3mmol of glutaric anhydride, and react at room temperature for 10h. The reaction solution was washed three times with 10% hydrochloric acid, and the dichloromethane layer was concentrated to obtain a light yellow liquid. Column chromatography with petroleum ether: ethyl acetate = 8:1 (V:V) gave a colorless liquid product with a yield of 64%.
[0085] 1 H NMR (CDCl 3,300MHz)δ:1.01(s,3H),1.42-1.68(m,6H),1.71(s,3H),1.93-2.04(m,4H),2.45(t,J=7.2Hz,4H),4.60 (s,3H),4.83(s,1H),4.88(s,1H),4.92(d,J=4.1Hz,1H),5.01(s,1H),5.04(s,1H),5.81(dd, J 1 =17.8Hz,J 2 =10.4Hz,1H).
[0086] 13 C NMR (CDCl 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com