A kind of benzimidazole benzaldehyde acetal p-phenylenediamine bis-Schiff base and preparation method thereof
A technology of formaldehyde condensing p-phenylenediamine and benzimidazole, applied in the field of benzimidazole benzaldehyde condensing p-phenylenediamine bis-Schiff base and its preparation, can solve the problems such as no bis-Schiff base reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
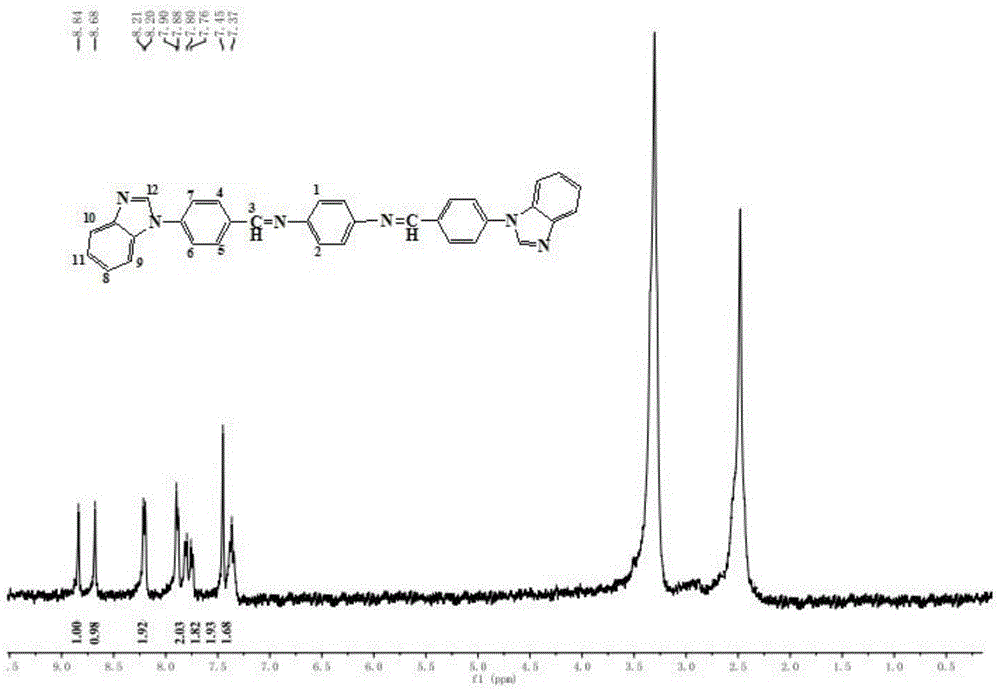
[0032] Weigh 1.180g (10mmol) of benzimidazole, weigh 1.240g (10mmol) of p-fluorobenzaldehyde and 1.380g (10mmol) of anhydrous potassium carbonate, dissolve in 30mL of dimethyl sulfoxide, add to the thermometer, A 50mL four-necked flask with a stirring device. Stir at a constant temperature at 90°C for 3 hours. After cooling to room temperature, extract with ethyl acetate for 3 times, wash with water, dry with anhydrous sodium sulfate, and spin-evaporate to obtain a yellow solid. Recrystallize with ethanol to obtain pure product. Dry in vacuum to obtain 4-benzene And imidazolyl benzaldehyde.
[0033] Weigh 0.222g (1mmol) of 4-benzimidazolylbenzaldehyde, weigh out 0.054g (0.500mmol) of p-phenylenediamine, dissolve it in 5mL of dimethyl sulfoxide, add to 10mL equipped with a thermometer and a stirring device Single-necked flask. Then add 0.120g (2mmol) of acetic acid to the above mixture, react at a constant temperature of 25°C for 10 hours, and distill under reduced pressure to r...
Embodiment 2
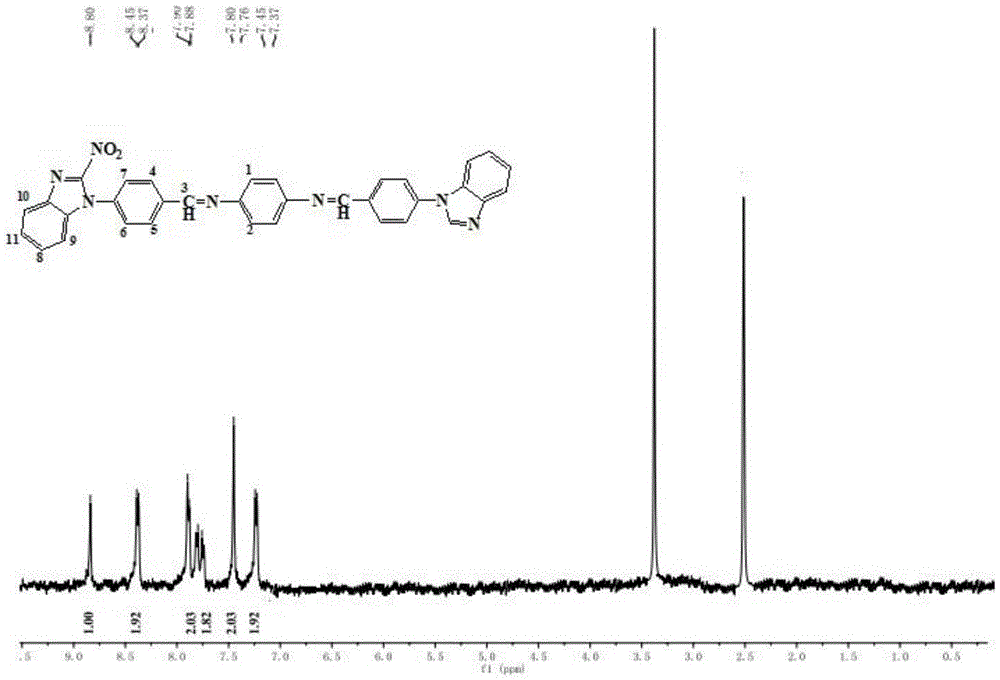
[0041] Weigh 3.260g (20mmol) of 5-nitrobenzimidazole, weigh 1.240g (10mmol) of p-fluorobenzaldehyde and 2.760g (20mmol) of anhydrous potassium carbonate, dissolve them in 35mL of dimethyl sulfoxide, and add to A 100 mL four-necked flask equipped with a thermometer and a stirring device. Stir at a constant temperature at 110°C for 2 hours. After cooling to room temperature, extract 3 times with ethyl acetate, wash with water, dry with anhydrous sodium sulfate, and spin-evaporate to obtain a yellow solid, which is recrystallized with ethanol to obtain a pure product, which is dried in vacuum to obtain 4-( 2-nitro-1-benzimidazolyl)benzaldehyde.
[0042] Weigh 0.267g (1mmol) of 4-(2-nitro-1-benzimidazolyl)benzaldehyde, weigh out 0.027g (0.25mmol) of p-phenylenediamine, dissolve it in 3mL of dimethyl sulfoxide, and add Into a 10-mL single-neck flask equipped with a thermometer and a stirring device. Then add 0.180g (3mmol) of acetic acid to the above mixture, react at a constant tem...
Embodiment 3
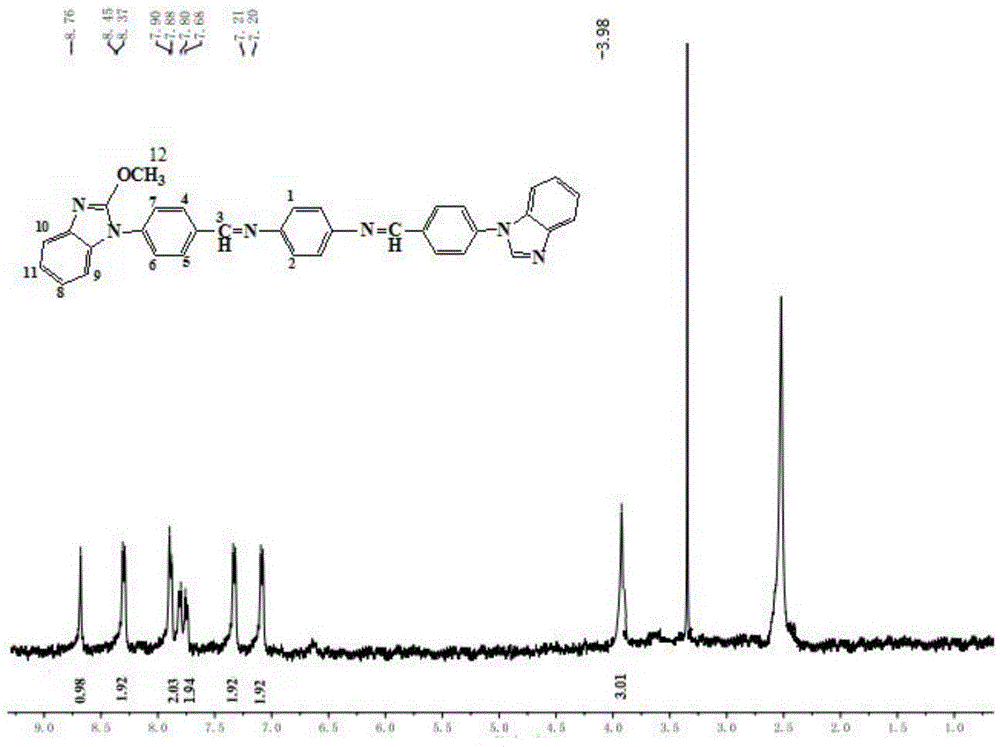
[0050] Weigh 2.960 g (20 mmol) of 2-methoxybenzimidazole, weigh 1.860 g (15 mmol) of p-fluorobenzaldehyde and 4.140 g (30 mmol) of anhydrous potassium carbonate, dissolve them in 40 mL of dimethyl sulfoxide, and add Into a 100mL four-neck flask equipped with a thermometer and a stirring device. Stir at a constant temperature at 120°C for 1 hour. After cooling to room temperature, extract 3 times with ethyl acetate, wash with water, dry with anhydrous sodium sulfate, and spin-evaporate to obtain a yellow solid, which is recrystallized with ethanol to obtain a pure product, which is dried in vacuum to obtain 4-( 2-Methoxy-1-benzimidazolyl)benzaldehyde.
[0051] Weigh out 0.756 g (3 mmol) of 4-(2-methoxy-1-benzimidazolyl)benzaldehyde, weigh out 0.130 g (1.250 mmol) of p-phenylenediamine, and dissolve it in 3 mL of dimethyl sulfoxide. Add to a 10-mL single-neck flask equipped with a thermometer and a stirring device. Then add 0.720g (12mmol) of acetic acid to the above mixture, rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com