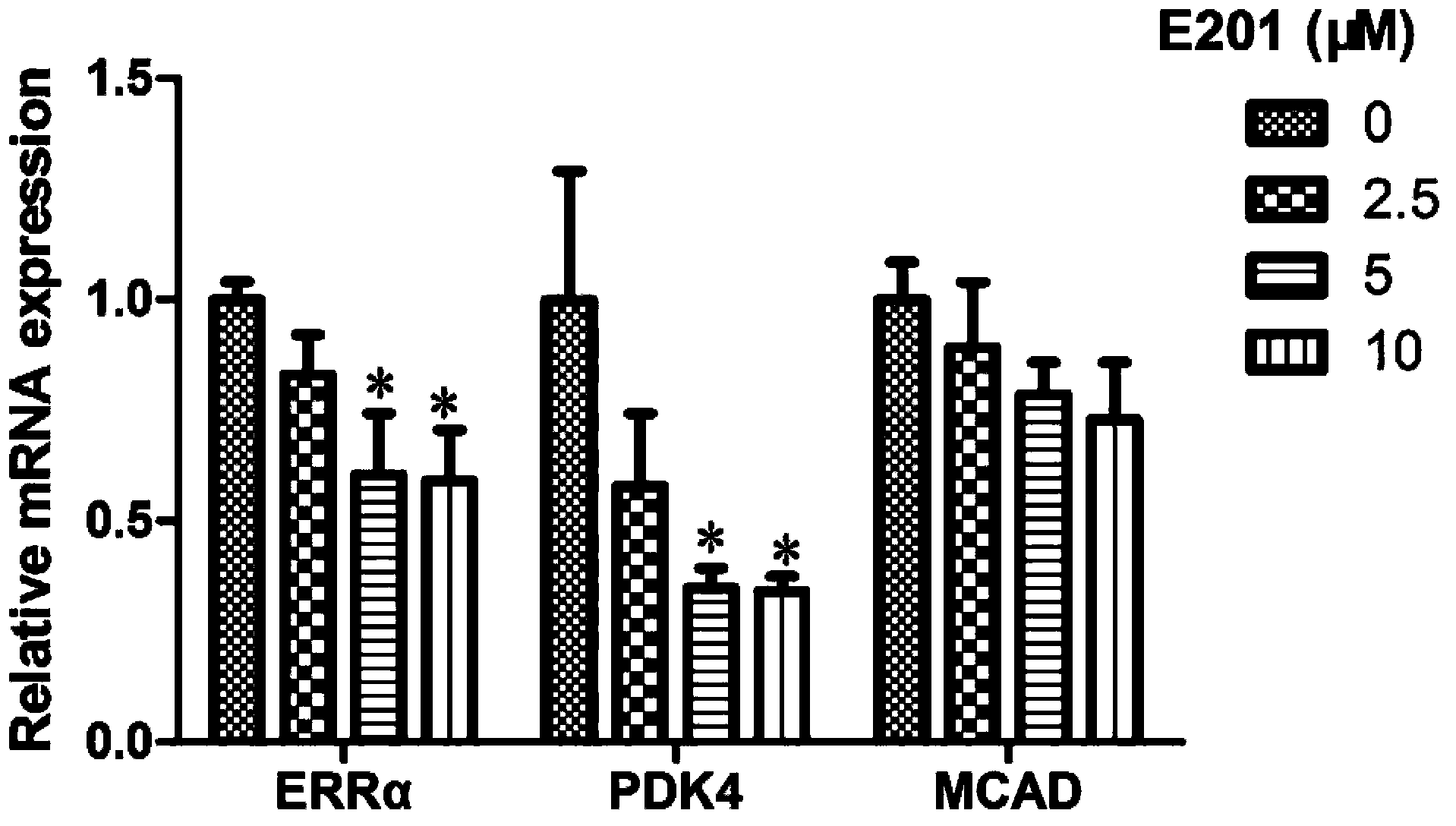
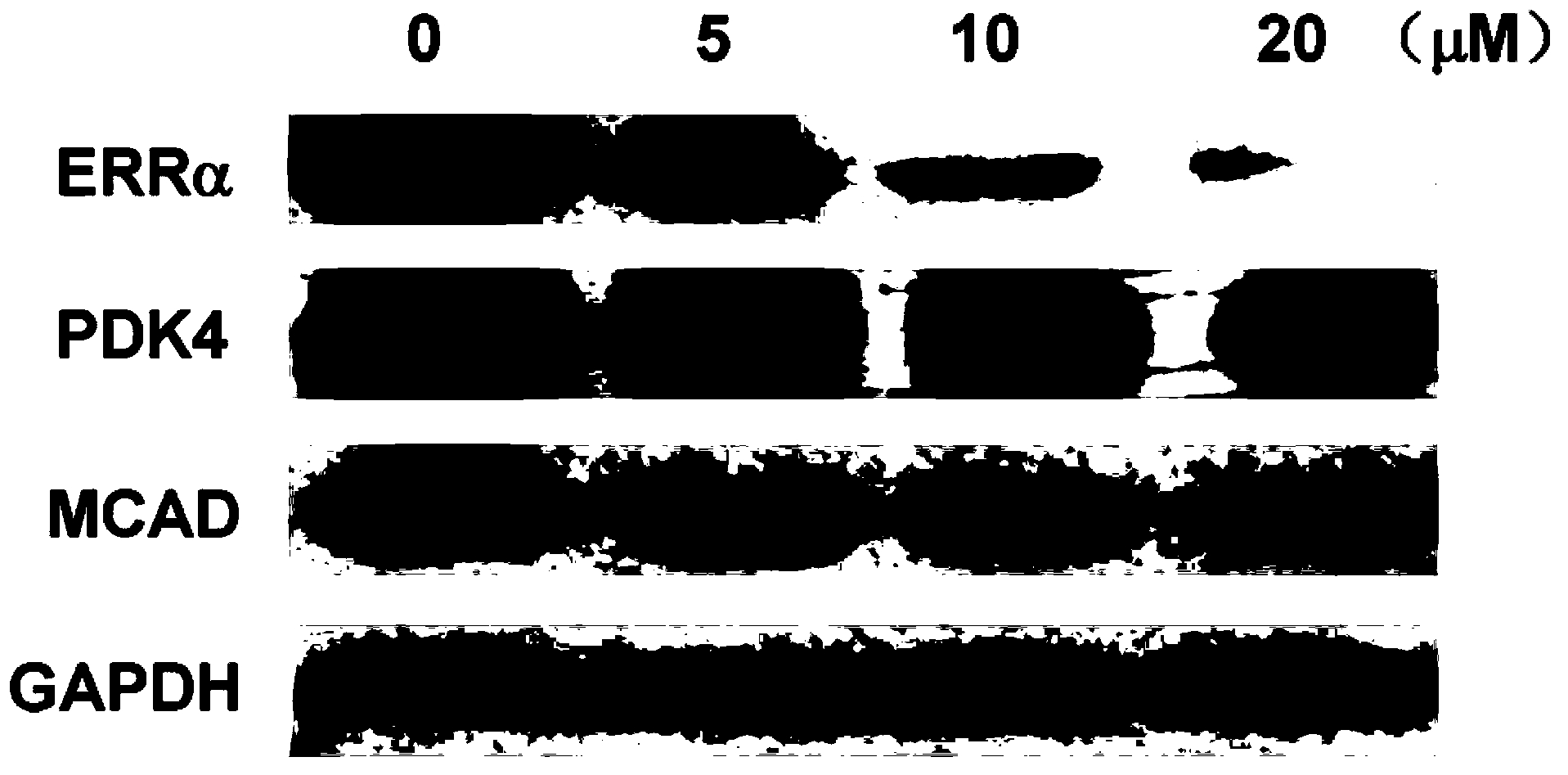
Compound used as estrogen-related receptor regulator and application of compound
A compound, triazole technology, applied in the field of medicinal chemistry, can solve problems such as no treatment means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] (4-Hydroxyphenyl)(1-(4-isopropylphenyl)-1-hydrogen-1,2,3-triazol-4-yl)methanone (E050)
[0230] (4-hydroxyphenyl)(1-(4-isopropylphenyl)-1H-1,2,3-triazol-4-yl)methanone
[0231]
[0232] Step a. 4-(tetrahydro-2-hydro-pyran-2-oxyl)benzaldehyde (2)
[0233] 4-(tetrahydro-2H-pyran-2-yloxy)benzaldehyde
[0234] At room temperature, 4-hydroxybenzaldehyde (1) (5g, 40.9mmol) was dissolved in anhydrous dichloromethane, protected by argon, and then 3,4-dihydro-2H-pyran (5.6mL, 61.4mmol ) and pyridinium p-toluenesulfonate (0.5g, 2mmol). Stir overnight at room temperature, and the reaction solution is successively washed with saturated NaHCO 3 solution and saturated brine three times, anhydrous Na 2 SO 4 Dried, then filtered and spin-dried, and separated by column chromatography to obtain 8.3 g (98%) of the product.
[0235] 1 HNMR(400MHz, CDCl3)δ9.89(s,1H),7.85-7.81(m,2H),7.17-7.15(m,2H),5.55-5.53(m,1H),3.88-3.82(m,1H) ,3.66-3.61(m,1H),2.05-1.96(m,1H),1.91-1.87(m,2H),1....
Embodiment 2
[0258] (3-Hydroxyphenyl)(1-(4-isopropylphenyl)-1-hydrogen-1,2,3-triazol-4-yl)methanone (E094)
[0259] (3-hydroxyphenyl)(1-(4-isopropylphenyl)-1H-1,2,3-triazol-4-yl)methanone
[0260]
[0261] The synthesis method is as in Example 1.
[0262] 1 HNMR (400MHz, DMSO-d 6 )δ9.86(s,1H),9.50(s,1H),7.93(d,J=8.4Hz,2H),7.74(d,J=7.6Hz,1H),7.68-7.67(m,1H), 7.51(d,J=8.4Hz,2H),7.41(t,J=8.0Hz,1H),7.12-7.09(m,1H),3.06-2.95(m,1H),1.25(d,J=6.8Hz ,6H).
[0263] 13 CNMR (125MHz, DMSO-d 6 )δ185.34, 157.83, 150.31, 147.60, 138.15, 134.48, 130.10, 128.25, 128.13, 121.37, 121.22, 120.96, 116.75, 33.54, 24.11.
[0264] HRMS(ESI):308.1388[M+H] + .
Embodiment 3
[0266] (2-Hydroxyphenyl)(1-(4-isopropylphenyl)-1-hydrogen-1,2,3-triazol-4-yl)methanone (E110)
[0267] (2-hydroxyphenyl)(1-(4-isopropylphenyl)-1H-1,2,3-triazol-4-yl)methanone
[0268]
[0269] The synthesis method is as in Example 1.
[0270] 1 HNMR (400MHz, DMSO-d 6 )δ11.40(s,1H),9.53(s,1H),8.39(dd,J=1.6,8.0Hz,1H),7.93(d,J=8.4Hz,2H),7.59-7.55(m,1H ),7.50(d,J=8.4Hz,2H),7.05-7.02(m,2H),3.06-2.95(m,1H),1.25(d,J=7.2Hz,6H).
[0271] 13 CNMR (125MHz, DMSO-d 6 )δ188.26, 160.29, 149.90, 147.26, 135.47, 133.96, 131.94, 127.85, 127.69, 121.59, 120.75, 119.13, 117.42, 33.08, 23.64.
[0272] HRMS(ESI):308.1393[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com