Catalytic oxygen production system containing duplex pyridine ruthenium (II) complex, preparation method of duplex pyridine ruthenium (II) complex and oxygen production method
A technology of ruthenium bipyridine and complexes is applied in the field of oxygen production in catalytic systems, and can solve the problems of poor catalyst stability, catalyst deactivation, and no Ca atoms obtained.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
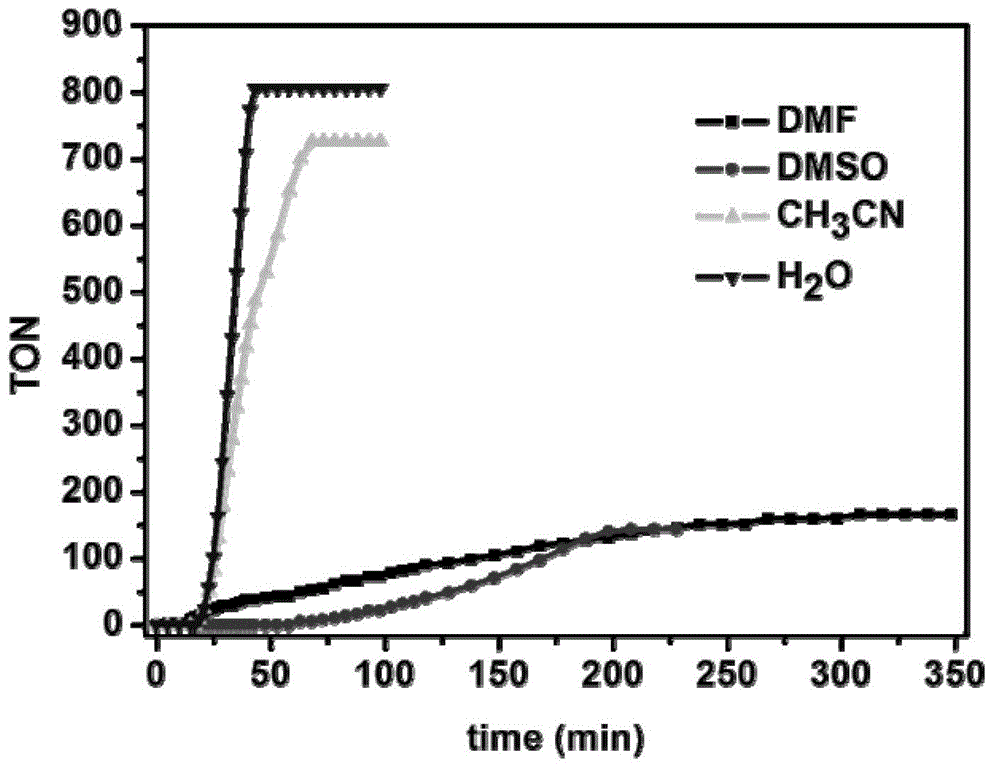
[0058] A catalytic oxygen generation system containing bipyridyl ruthenium (Ⅱ) complex: the concentration of bipyridyl ruthenium (Ⅱ) complex is 2.0×10 -4 mol / L; the concentration of ammonium cerium nitrate is 0.6mol / L; the concentration of trifluoromethanesulfonic acid is 0.2mol / L; the solvent is water; the total sample volume is 4mL.
[0059]The method of preparing oxygen by using the above-mentioned thermal catalytic oxygen generation system is: stir the sample at room temperature, and monitor the amount of oxygen generated in the sample by the drainage method; after stirring for 4 hours, the calculated oxygen production of the sample is about 17.6mL (TON=979) ; Indicating that the system was stable during the first 4 hours of reaction.
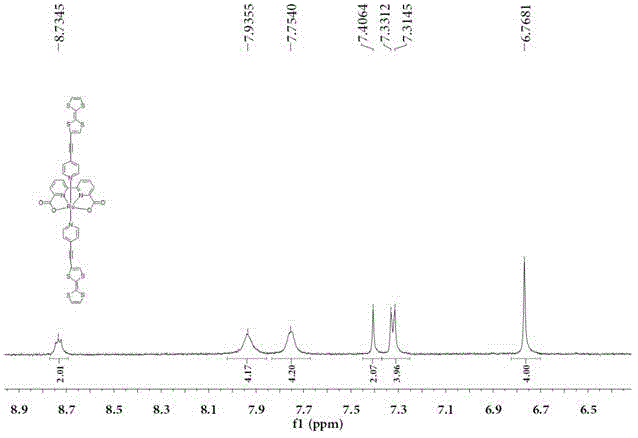
[0060] The bipyridine ruthenium (II) complex is a compound with the following molecular formula (F2):
[0061]
Embodiment 2
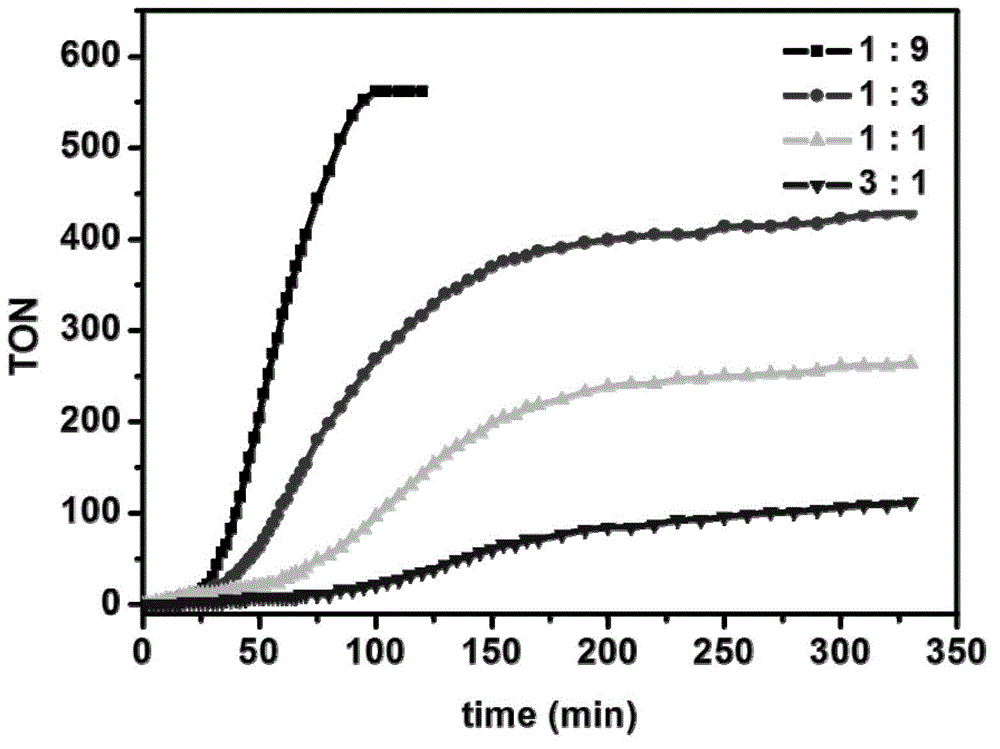
[0063] A catalytic oxygen generation system containing bipyridyl ruthenium (Ⅱ) complex: the concentration of bipyridyl ruthenium (Ⅱ) complex is 2.0×10 -4 mol / L; the concentration of ceric ammonium nitrate is 0.6mol / L; the concentration of trifluoromethanesulfonic acid is 0.2mol / L; the solvent is water and acetonitrile; the volume ratio of water and acetonitrile is 9:1; the total sample volume is 4mL.
[0064] The method of preparing oxygen by using the above thermocatalytic oxygen generation system is: stir the sample at room temperature, and monitor the amount of oxygen generated in the sample by the drainage method; after stirring for 4 hours, the calculated oxygen production of the sample is about 15.6mL (TON=868) ; Indicating that the system was stable during the first 4 hours of reaction.
[0065] The bipyridyl ruthenium (II) complex and the bipyridyl ruthenium (II) complex in Example 1 are the same substance.
Embodiment 3
[0067] A catalytic oxygen generation system containing bipyridyl ruthenium (Ⅱ) complex: the concentration of bipyridyl ruthenium (Ⅱ) complex is 2.0×10 -4 mol / L; the concentration of ammonium cerium nitrate is 0.6mol / L; the concentration of trifluoromethanesulfonic acid is 0.2mol / L; the solvent is water and DMF; the volume ratio of water and DMF is 9:1; the total sample volume is 4mL.
[0068] The method of preparing oxygen by using the above thermocatalytic oxygen generation system is: stir the sample at room temperature, and monitor the amount of oxygen generated in the sample by the drainage method; after stirring for 5 hours, the calculated oxygen production of the sample is about 3.0mL (TON=166) ; Indicating that the system was stable during the first 5 hours of reaction.
[0069] The bipyridyl ruthenium (II) complex and the bipyridyl ruthenium (II) complex in Example 1 are the same substance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com