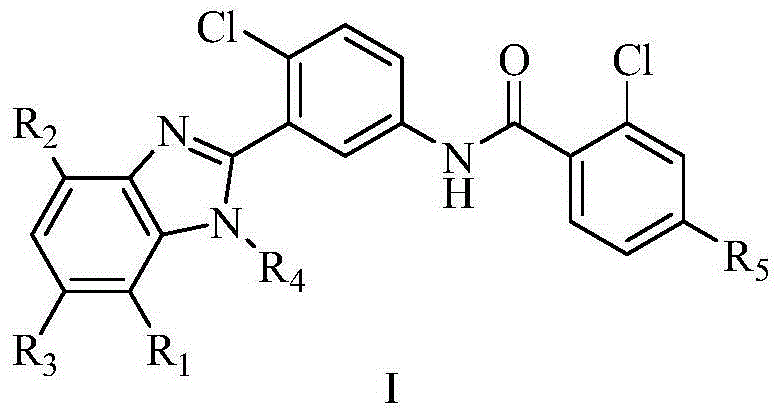
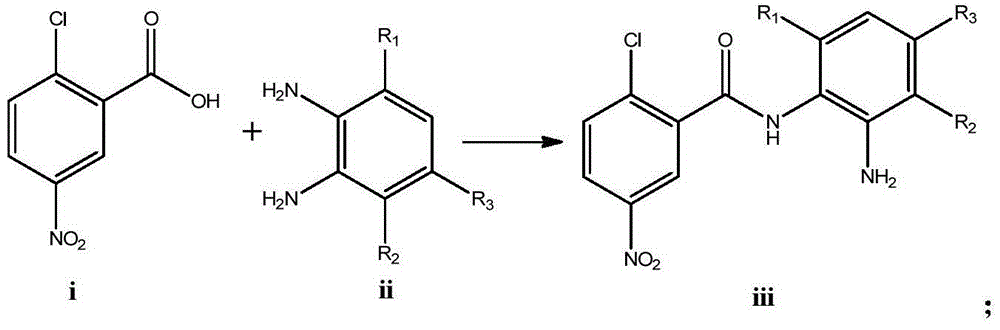
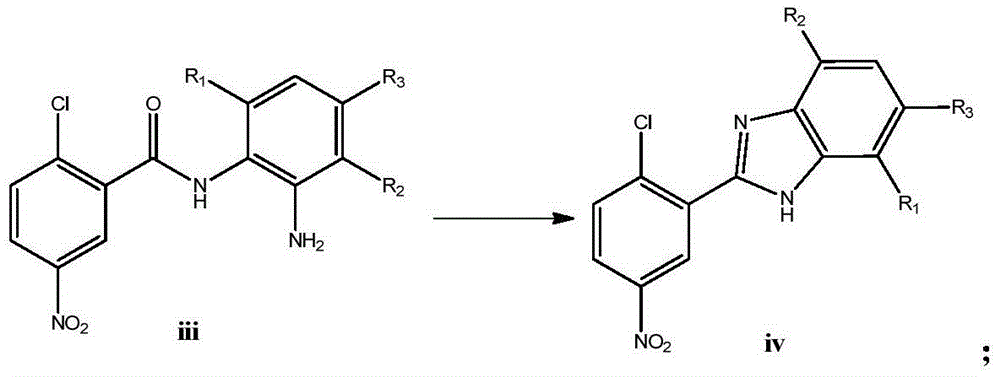
Diaryl amide compound containing benzimidazole group as well as synthesis and application of diaryl amide compound
A technology of benzimidazolyl and arylamide, applied in the field of chemical medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of 2-chloro-N-[4-chloro-3-(1H-benzimidazol-2-yl)phenyl]-4-thiamphenicol benzamide (Ⅰ-1)
[0053] ①Add 2.98mmol of compound ⅰ-1, 2.98mmol of o-phenylenediamine (compound ⅱ-1), 3.28mmol of HBTU, 5.96mmol of triethylamine and 20mL of THF into a 50mL pear-shaped flask, stir at room temperature for 5h under nitrogen protection, and react After adding 40 mL of water to the solution, CH 2 Cl 2 Extract three times, 30mL each time, combine the organic phases, wash with 30mL of water, wash with 30mL of saturated brine, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain the crude product, the crude product is purified on a silica gel column, and the mobile phase is petroleum ether: ethyl acetate: triethylamine = 1:1:0.005, 714 mg of product was obtained with a yield of 82%. ESI-MS:m / z292.05[M+H] + .
[0054]
[0055] ②Add 1.714mmol of the product obtained in step ① (compound Ⅲ-1) and 12mL HOAc in a 25mL pear-shaped bottle, stir and ref...
Embodiment 2
[0062] Synthesis of 2-chloro-N-[4-chloro-3-(1-methyl-1H-benzimidazol-2-yl)phenyl]-4-thiamphenicol benzamide (Ⅰ-2)
[0063] ①Add 2.98mmol of compound ⅰ-1, 2.98mmol of o-phenylenediamine (compound ⅱ-1), 3.28mmol of HBTU, 5.96mmol of triethylamine and 20mL of THF into a 50mL pear-shaped flask, stir at room temperature for 5h under nitrogen protection, and react After adding 40 mL of water to the solution, CH 2 Cl 2 Extract three times, 30mL each time, combine the organic phases, wash with 30mL of water, wash with 30mL of saturated brine, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain the crude product, the crude product is purified on a silica gel column, and the mobile phase is petroleum ether: ethyl acetate: triethylamine = 1:1:0.005, 714 mg of product was obtained with a yield of 82%. ESI-MS:m / z292.05[M+H] + .
[0064]
[0065] ②Add 1.714mmol of the product obtained in step ① (compound Ⅲ-1) and 12mL HOAc in a 25mL pear-shaped bottle, sti...
Embodiment 3
[0074] 2-Chloro-N-[4-chloro-3-(4,7-dimethoxy-1H-benzimidazol-2-yl)phenyl]-4-thiamphenicol benzamide (Ⅰ-3) Synthesis
[0075] The difference with embodiment 1 is:
[0076] The o-phenylenediamine of step ① in embodiment 1 is replaced by 3,6-dimethoxy o-phenylenediamine;
[0077] Compound I-3. 1 H NMR (400MHz, CDCl 3 )δ10.90(1H,s),8.66-8.53(2H,m),7.61-7.52(2H,m),7.31(1H,d,J=7.9Hz),7.18(1H,d,J=7.9Hz ), 6.45(2H,s), 3.84(6H,s), 2.89(3H,s); ESI-MS: m / z520.03[M+H] + .
[0078]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com