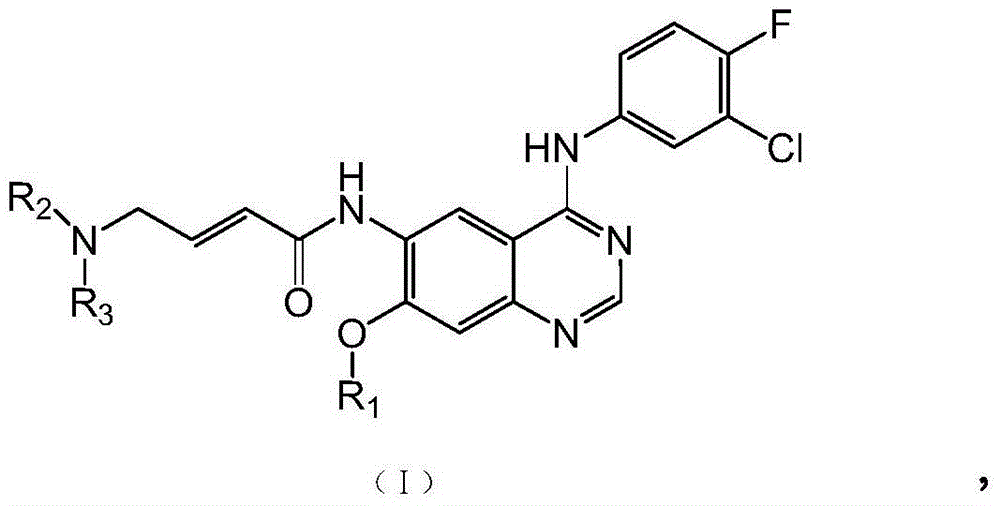
Quinazoline compound as well as preparation method and application thereof in preparing tyrosine kinase inhibitor
A technology of quinazolines and compounds, which is applied in the field of pharmaceutical preparation, can solve the problems of low conversion rate, low yield, unsuitability for industrial production of quinazoline crotyl compounds, etc., and achieve the effect of easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
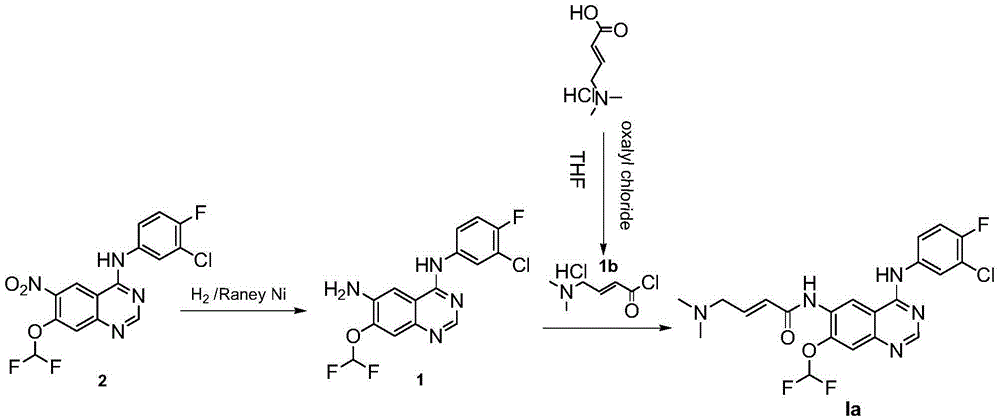
[0047] Example 1 Preparation of Compound IIIa by Catalytic Hydrogenation
[0048] 1. The reaction equation is as follows:
[0049]
[0050] 2. The feeding ratio is as follows:
[0051] Material name
molecular weight
Actual feeding amount
The molar ratio of
weight ratio
Compound IIa
384.70
600.0g
1
1
THF(1)
72.11
4.0kg
6.7
32.04
0.7kg
1.2
85.67
120.0g
0.9
0.2
53.49
60.0g
0.7
0.1
Saturated Salt Water (1)
/
4.2kg
7
Saturated Salt Water (2)
/
3.2kg
5.3
THF(2)
72.11
1000mL
0.75
[0052] 3. Process operation steps
[0053] Compound IIa was added to a 10L double-jacketed glass reactor, then THF (1) and methanol were added to the reactor, and the compound II was stirred to dissolve. Then add Raney nickel and ammoniu...
Embodiment 2
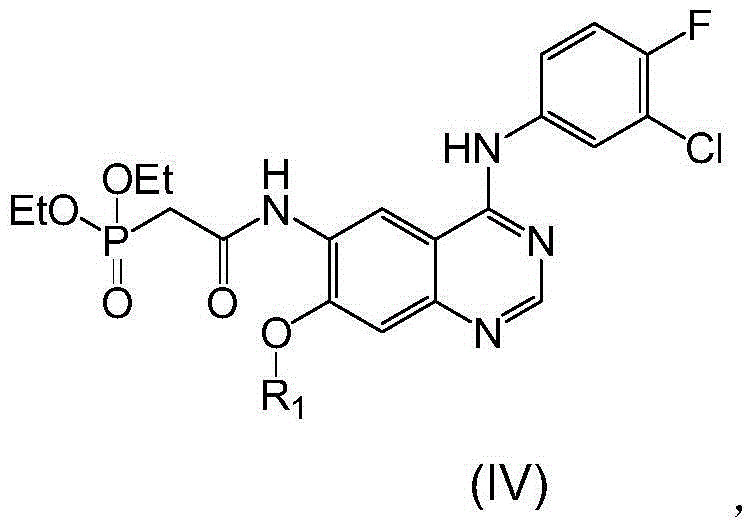
[0054] The preparation of embodiment 2 compound IVa
[0055] 1. The reaction equation is as follows:
[0056]
[0057] 2. The feeding ratio is as follows:
[0058] Material name
molecular weight
Actual feeding amount
The molar ratio of
weight ratio
Compound IIIa
354.71
516.5g
1
1
diethylphosphonoacetic acid
196.14
599.9g
2.1
1.2
N,N'-Carbonyldiimidazole (CDI)
162.15
495.9g
2.1
1.0
THF(1)
72.11
1.81kg
3.5
THF(2)
72.11
729.3g
1.4
THF(3)
72.11
2.29kg
4.4
Methyl tert-butyl ether (MTBE)
88.15
12.91kg
25
MTBE: THF mixture (1)
/
2804.8g
5.4
18
1452.7g
2.8
MTBE: THF mixture (2)
/
1874.9g
3.6
[0059] 3. Process operation steps
[0060] Add CDI into a 10L reaction kettle, then add THF (1), st...
Embodiment 3-7
[0068] Examples 3-7 respectively provide methods for preparing corresponding compound (IV) from different compounds (III), the steps are basically the same as in Example 2, and the conditions refer to Table 1 (for the preparation method of compound (III), refer to Example 1 of CN10283550A).
[0069] Table 1
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com