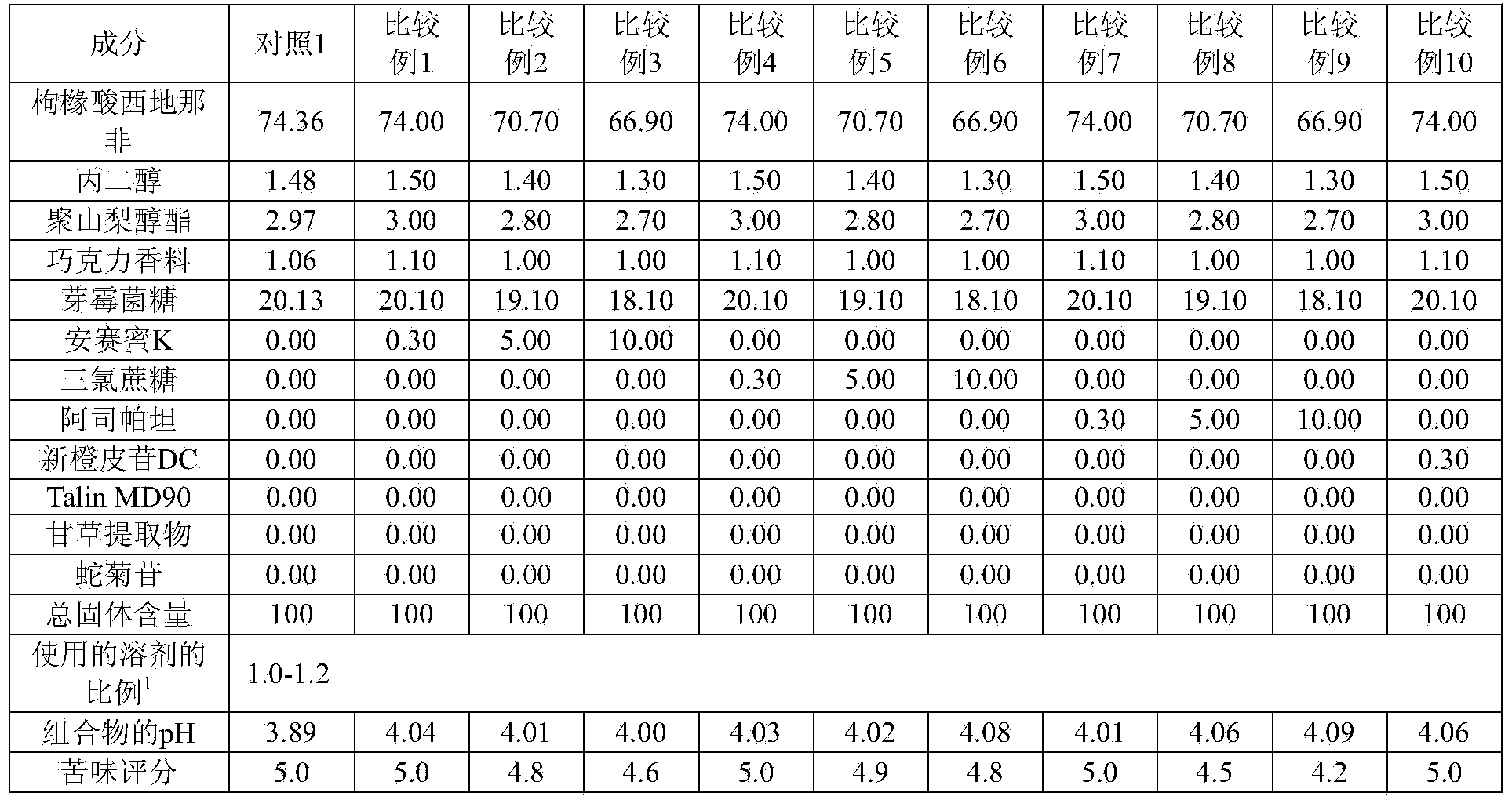High-content fast dissolving film with masking of bitter taste comprising sildenafil as active ingredient
A sildenafil and film-dissolving technology, which is applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as difficult production of fast-dissolving film preparations, and achieve masking of bitterness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-27
[0146] As shown in Tables 3 and 4, pharmaceutical compositions for oral film formulations were prepared, and their pH, bitterness score, and film-forming ability were evaluated.
[0147] [table 3]
[0148]
[0149] 1) The proportion of solvent used: the amount of solvent relative to the total solid content (multiple)
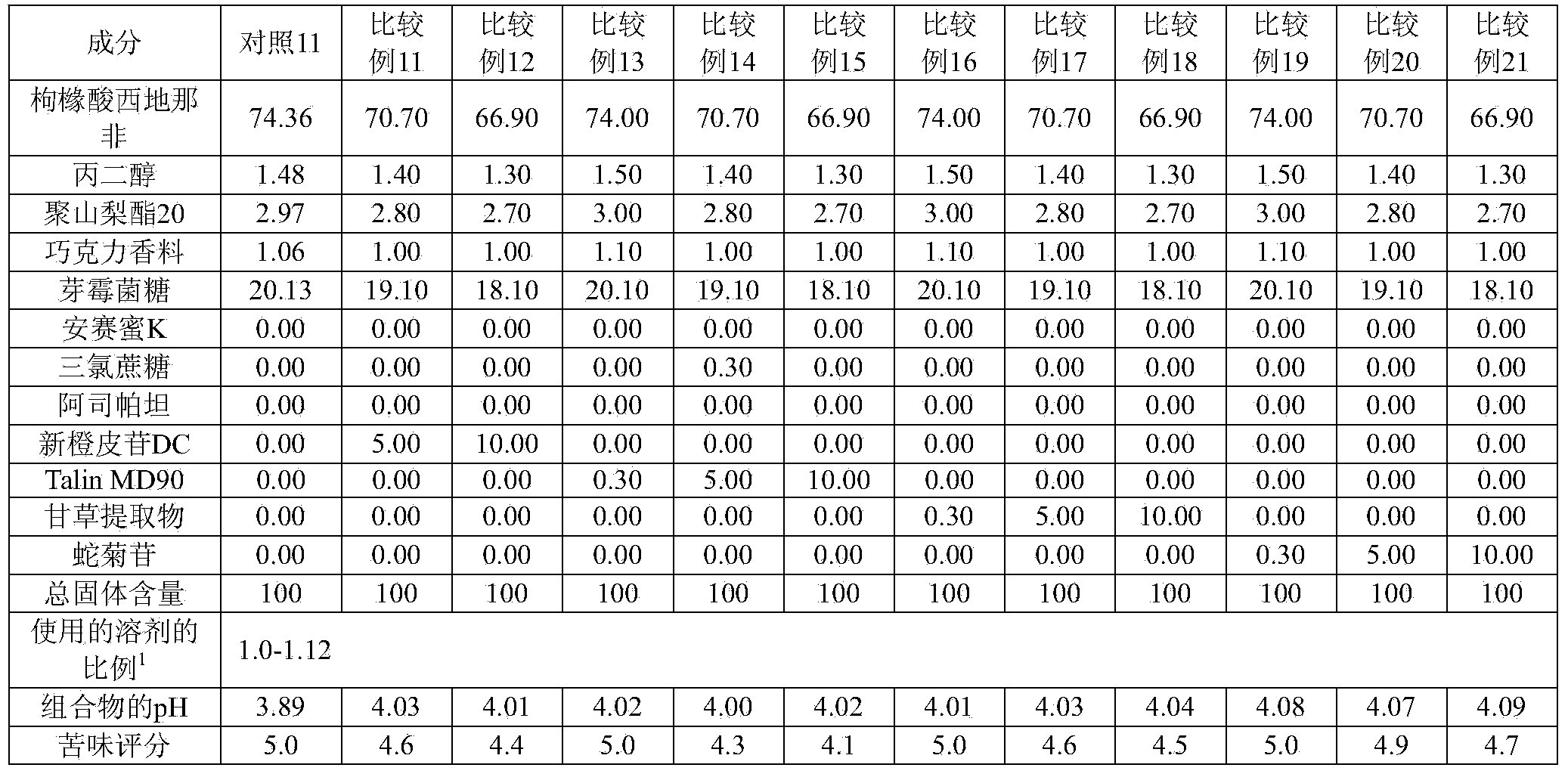
[0150] [Table 4]
[0151]
[0152]
[0153] 1) The proportion of solvent used: the amount of solvent relative to the total solid content (multiple)
[0154] As can be seen from the results in Tables 3 and 4, when the pH of the composition was adjusted to 4.41 or more (with a maximum pH of 5.81) using a single alkalizing agent, the bitterness was effectively masked but greatly deteriorated film-forming ability. Therefore, these compositions are not suitable for the production of oral film formulations.
Embodiment 28-40
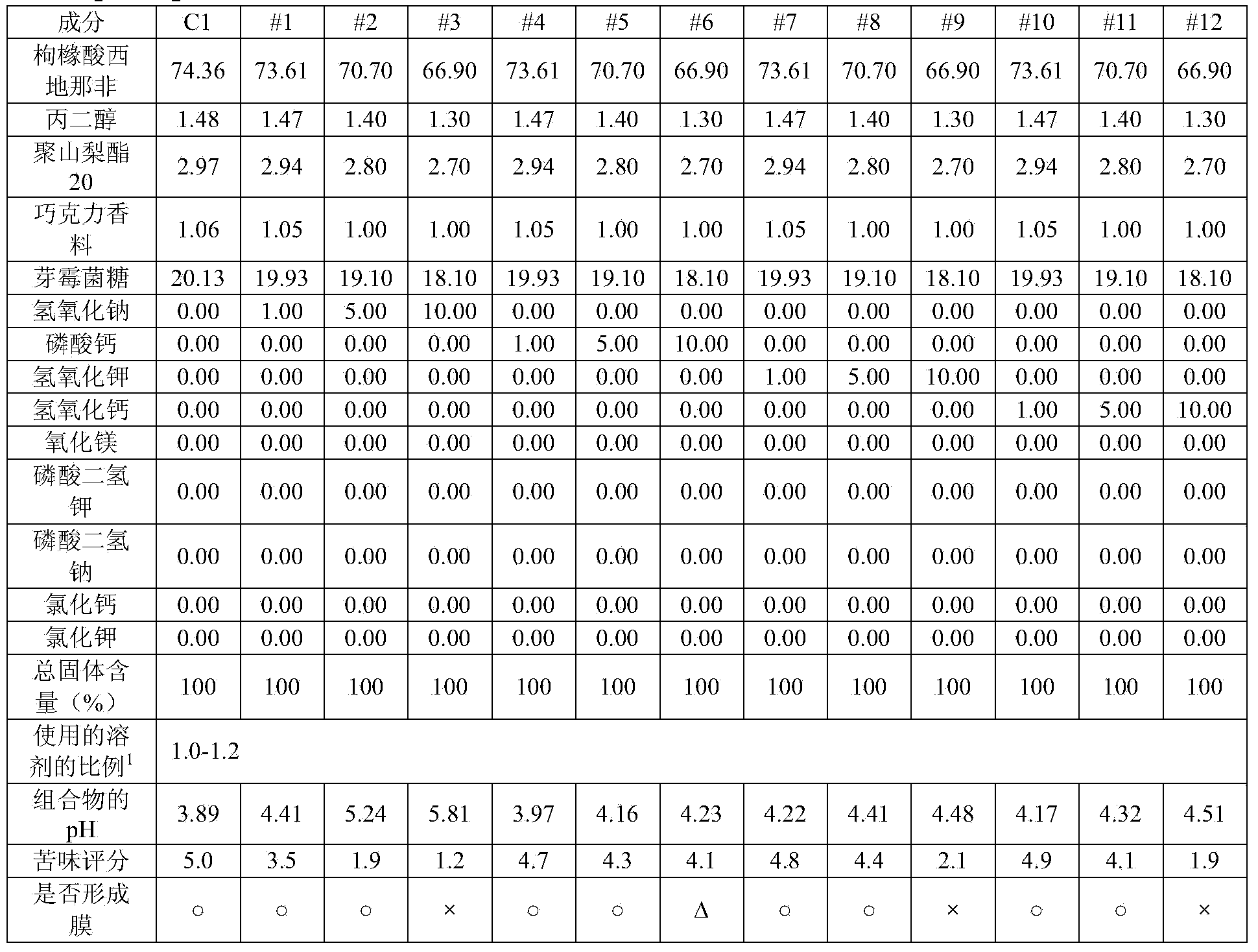
[0156] As shown in Table 5, pharmaceutical compositions for oral film formulations were prepared, and their pH, bitterness score, and film-forming ability were evaluated.
[0157] [table 5]
[0158]
[0159] 1) The proportion of solvent used: the amount of solvent relative to the total solid content (multiple)
[0160] As can be seen from the results in Table 5, when the combination of magnesium oxide and sodium hydroxide was used as the alkalizing agent, the bitter taste was effectively masked and good film-forming ability was obtained.
Embodiment 41-44
[0162] As shown in Table 6, pharmaceutical compositions for oral film formulations were prepared, and their pH, bitterness score, and film-forming ability were evaluated.
[0163] [Table 6]
[0164]
[0165] 1) The proportion of solvent used: the amount of solvent relative to the total solid content (multiple)
[0166] As can be seen from the results in Table 6, when the combination of magnesium oxide and sodium hydroxide was mixed with Talin MD90 and aspartame in a predetermined ratio as an alkalizing agent, the bitter taste was effectively masked and good results were obtained. film ability and gives a very comfortable feeling when taking it.
[0167] 2. Group B
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com