Single domain antibodies as inhibitors of PCSK9
An antibody, CDR2 technology, applied in the direction of antibodies, anti-enzyme immunoglobulin, biochemical equipment and methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
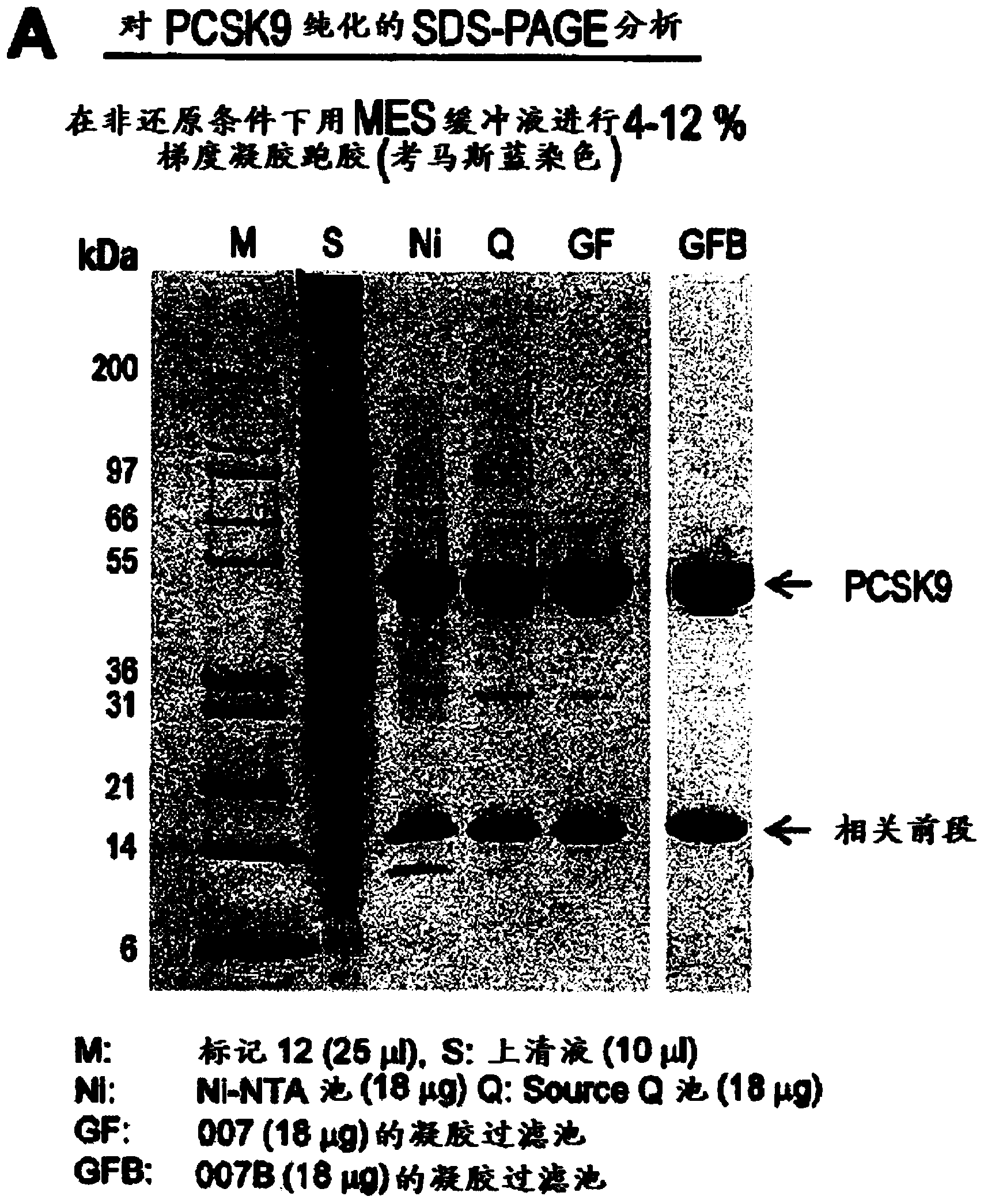
[0552] Example 1: Production and purification of recombinant PCSK9 protein
[0553] From 35L Baculovirus High Five TM Cells produce a large amount of PCSK9-(His) 6 and it was subjected to multiple purification steps, sequentially including Ni 2+ Affinity purification followed by mono-Q TM Anion exchange and finally gel filtration chromatography. The results of Coomassie blue staining (Coomassie blue staining) of 18 μg of total protein separated from each preparation under non-reducing conditions in MES buffer SDS-PAGE with a gradient of 4-12% are shown in figure 1 In A. Location of molecular size markers (M); High Five prior to purification TM Total protein in supernatant (S); by Ni 2+ Affinity Chromatography (Ni), SOURCETM 15Q Anion Exchange (Q) and Superdex TM 200 gel filtration (GF) for purification. M: Marker 12 (25 μl); S, supernatant (10 μl); Ni, Ni-NTA pool (18 μg); Q, SOURCE Q pool (18 μg); GF, gel filtration pool from batch 7 (18 μg), GFB: Gel Filtration Ce...
Embodiment 2
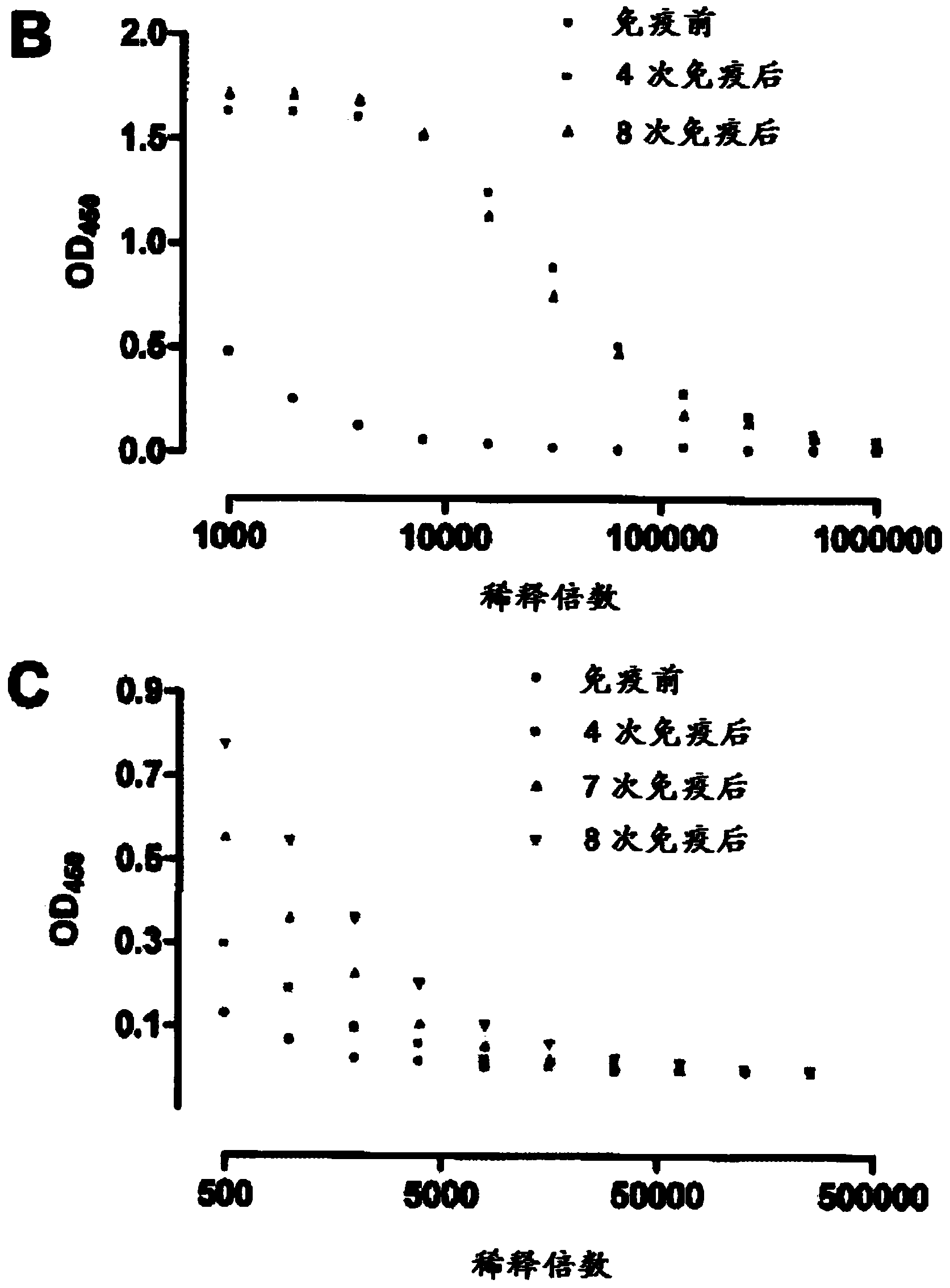
[0554] Example 2: Immunization of llamas with purified PCSK9 protein
[0555] Isolation from immune phage display libraries is the easiest way to generate high affinity sdAbs. This involves immunizing llamas with antigen; monitoring the immune response; constructing phage display libraries; and subsequent phage display panning. Several injections of a human purified PCSK9 heterodimeric complex formed by the prosegment (amino acids 31 to 152) and the catalytic subunit (amino acids 153 to 692) were used to immunize llamas. The immune response was monitored and characterized using reactivity against PCSK9. Figure 1B and 1 C shows the immune response of the llama. While in [B] the llama immune response is evident after 4 and 8 immunizations, in [C] the results show 4 and 8 immunizations when lower concentrations of antigen (PCSK9) are used There is indeed a difference - indicating that very high affinity Abs may be present after 8 weeks of immunization. After immunization of...
Embodiment 3
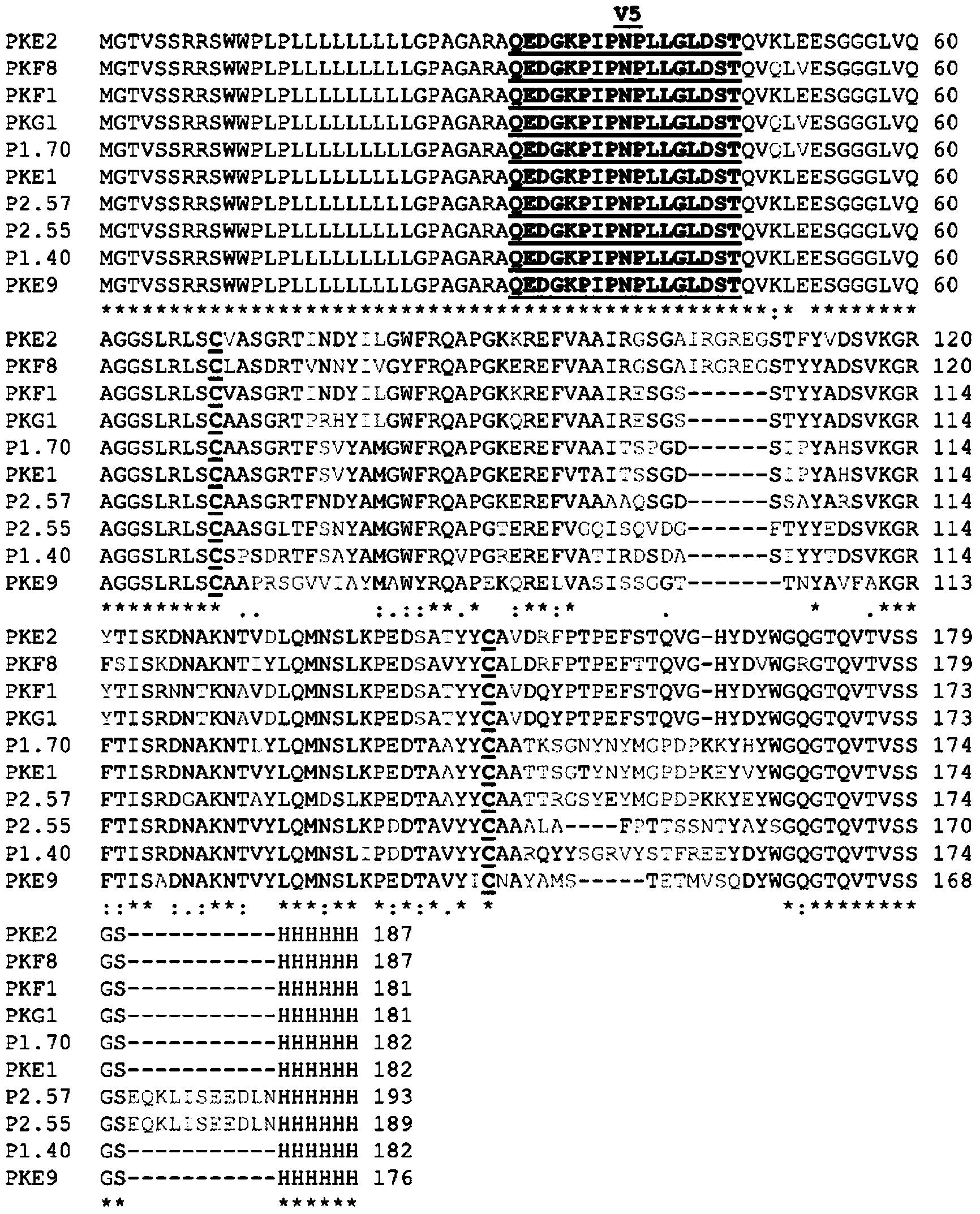
[0556] Example 3: Screening for Vs specific for PSCK9 H H domain (sdAb)
[0557] Use V to camel IgG H / V H Primer pairs specific for both H and hinge regions encode V H and V H H DNA was amplified. will V H The H segment is based on its size relative to the V H separate. Use pair V H H Specific primers amplify V H H gene and clone it into a phagemid-based phage display vector. More specifically, three different sense primers (termed J' and corresponding to the 5' end of IgG) were used (including MJ1 (GCCCAGCCGGCCATGG CCSMKGTGCAGCTGGTGGAKTCTGGGGGA (SEQ ID NO: 144)), MJ2 (CAGCCGGCCATGGCCCAGGTAAAGCTGGAGGAGTCT GGGGGA (SEQ ID NO: 145)) and MJ3 (GCCCAGCCGGCCATGG CCCAGGCTCAGGTACAGCTGGTGGAGTCT (SEQ ID NO: 146))) and two antisense primers corresponding to the CH2 domain DNA sequence (CH2 (CGCCATCA AGGTACCAGTTGA (SEQ ID NO: 147)) and CH2b3 (GGGGTACCTG TCATCCACGGACCAGCTGA ( SEQ ID NO:148)) to amplify the VH-CH1-hinge-CH2 region or V of conventional IgG H H-Hinge-CH2. The app...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com