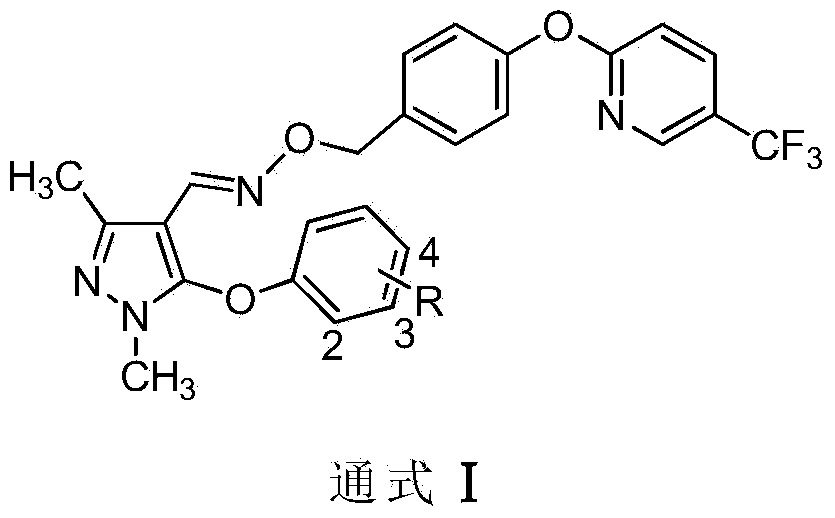
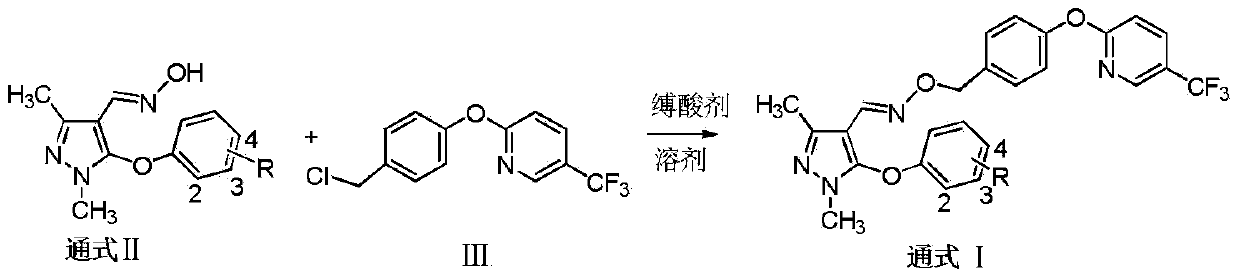
Preparation and application of pyrazole oxime ether compound containing trifluoro methyl pyridine
A technology of trifluoromethylpyridine and pyrazole oxime ether, which is applied in the field of pesticides to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of Compound Ia (R=2,3-F 2 )
[0035]
[0036] Add 5mmol of pyrazole oxime intermediate IIa, 5mmol of 2-(4-chloromethyl)-phenoxy-5-trifluoromethylpyridine and 20mL of acetonitrile in the reaction flask, drop 7.5mmol of cesium carbonate into it under stirring, and The resulting mixture was heated to reflux for 16 hours. Turn off heat. Filtrate, concentrate the mother liquor to dryness, mix the sample with silica gel and load it into a column for separation and purification to obtain the target compound Ia. White solid, yield 43.6%, 1 H NMR (400 MHz, CDCl 3 ): δ8.45(s,1H,Py-H),7.91(d,J=8.8Hz,1H,Py-H),7.85(s,1H,CH=N),7.36(d,J=8.0Hz ,2H,Ar-H and Py-H),7.11(d,J=8.0Hz,2H,Ar-H),7.02(d,J=8.4Hz,1H,Ar-H),6.93~6.97(m, 2H, ArH), 6.54(d, J=8.4Hz, 1H, Ar-H), 5.00(s, 2H, CH 2 ),3.68(s,3H,N-CH 3 ),2.36(s,3H,CH 3 ).
Embodiment 2
[0038] Synthesis of compound Ib (referring to the synthesis of compound Ia, wherein R=4-CH 3 )
[0039]
[0040] Add 3 mmol of pyrazole oxime intermediate IIb, 2.7 mmol of 2-(4-chloromethyl)-phenoxy-5-trifluoromethylpyridine intermediate III and 20 mL of DMF into the reaction flask, and add 8 mmol of carbonic acid into it under stirring. Potassium, the resulting mixture was heated to reflux for 20 hours. Turn off heat. After filtration, the mother liquor was concentrated to dryness, mixed with silica gel and packed into a column for separation and purification to obtain the target substance Ib. White solid, yield 50.5%, 1 H NMR (400MHz, CDCl 3 ): δ8.46(s,1H,Py-H),7.90(d,J=8.4Hz,1H,Py-H),7.84(s,1H,CH=N),7.39(d,J=7.6Hz ,2H,Ar-H and Py-H),7.12(d,J=7.2Hz,4H,Ar-H),7.01(d,J=8.4Hz,1H,Ar-H),6.81(d,J= 7.6Hz, 2H, Ar-H), 5.05(s, 2H, CH2), 3.61(s, 3H, N-CH 3 ),2.40(s,3H,CH 3 ),2.32(s,3H,CH 3 ).
Embodiment 3
[0042] Synthesis of compound Ic (referring to the synthesis of compound Ia, wherein R=H)
[0043]
[0044] Add 2 mmol of pyrazole oxime intermediate IIc, 2.2 mol of 2-(4-chloromethyl)-phenoxy-5-trifluoromethylpyridine intermediate III and 30 mL of DMSO into the reaction flask, and add 10 mmol of di isopropylethylamine (DIEA), and the resulting mixture was heated to 100° C. for 10 hours. Turn off heat. Filtration, concentrating the mother liquor to dryness, mixing with silica gel and packing into a column for separation and purification to obtain the target object Ic, a white solid, with a yield of 43.8%. 1 H NMR (400MHz, CDCl 3):δ8.46(s,1H,Py-H),7.91(d,J=8.4Hz,1H,Py-H),7.84(s,1H,CH=N),7.34~7.39(m,4H, Py-H and ArH), 7.01~7.12(m, 4H, ArH), 6.92(d, J=7.6Hz, 2H, Ar-H), 5.03(s, 2H, CH 2 ),3.63(s,3H,N-CH 3 ),2.40(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com